Complications of fluid overload during hysteroscopic surgery: cardiomyopathy and epistaxis - A case report -
Article information
Abstract
Background
Hysteroscopic surgery has been used in various gynecological fields. However, massive fluid overload can occur as a complication due to persistent infusion of media for uterine cavity distension. We present the case of a woman who developed cardiomyopathy with pulmonary edema and epistaxis during hysteroscopic surgery.
Case
A 76-year-old female underwent hysteroscopic septectomy. She manifested abrupt, active nasal bleeding and regurgitation in the intravenous line. Heart rate, SpO2, and PETCO2 decreased from 55 beats/min to 29 beats/min, from 100% to 56%, and from 31 mmHg to 9 mmHg, respectively. After the operation, brain CT showed bilateral prominent superior ophthalmic vein dilation. Echocardiography showed left ventricle apical ballooning and global hypokinesia. The patient recovered after two days of conservative management, with no sequelae.
Conclusions
Although hysteroscopic surgery is a simple procedure, careful monitoring is necessary to prevent complications from absorption of fluid distending media during the procedure.
Hysteroscopic surgery is widely used for treatment of gynecological diseases, such as septae, synechiae, and intrauterine myoma. Advantages include less invasiveness and faster recovery compared to traditional laparotomy. It is reported that the incidence of complication is about 0.22% [1]. Known complications include uterine perforation, massive bleeding, and fluid overload [1]. Fluid overload can occur because of continuous infusion of fluid media for distending the uterus and can be accompanied by severe complications, such as pulmonary edema, hyponatremia, hypo-osmolarity, and cerebral edema. If such complications are not recognized, pulmonary edema and cardiovascular collapse may also occur and can lead to catastrophic outcomes [2]. Direct media absorption through an exposed vessel or sinus during surgery is the main cause of fluid overload [3]. However, it is uncommon to report cardiomyopathy as well as pulmonary edema due to fluid overload. Herein, we report a case of severe bradycardia, pulmonary edema, cardiomyopathy, and epistaxis with extreme hyponatremia during hysteroscopic surgery.
CASE REPORT
This case report was approved by the Wonju Severance Christian Hospital Institutional Review Board (IRB). The IRB approval number is CR319331.
A 76-year-old female patient, 149 cm in height and 62 kg in weight, was scheduled for laparoscopic surgery on a calcified mass, 3.1 cm in size, located on the rectovesical pouch. She was also scheduled for a hysteroscopy due to intrauterine septae.
The patient had a medical history of diabetes mellitus and hyperthyroidism. Upon arrival in the operating room, blood pressure was 162/61 mmHg, heart rate was 75 beats/min, and SpO2 was 99%. Anesthesia was induced with propofol 100 mg and rocuronium 50 mg. After tracheal intubation, anesthesia was maintained with an oxygen-air-desflurane mixture and a remifentanil infusion.
Laparoscopic myomectomy was performed for 15 min at about 15° in the Trendelenburg position. Subsequently, the patient’s posture was changed to the lithotomy position, and hysteroscopy was performed. Hysteroscopy-guided septectomy was carried out with a monopolar instrument. The distending fluid media was infused with an inflated pressure cuff registered at a pressure of 100 mmHg.
Fifty minutes after hysteroscopic surgery, the pulse rate of the patient suddenly decreased from 50–60 beats/min to 29 beats/min. Peak airway pressure increased from 19 mmHg to 35 mmHg. SpO2 decreased gradually from 100% to 85% and then to 56% over the span of 20 min. PETCO2 decreased from 31 mmHg to 16 mmHg and then to 9 mmHg. Blood pressure was maintained at 90–105/60–70 mmHg. We changed the oxygen concentration of the ventilator from 0.5 to 1.0. The pulse rate was recovered to 60 beats/min after intravenous atropine 0.5 mg injection. Non-invasive blood pressure was maintained at the pre-event status, so we did not inject any inotropic agents. A pink and frothy secretion was observed upon tracheal suction. Spontaneous nasal bleeding in both nostrils, whole-face congestion, and IV line regurgitation in the left arm occurred. We immediately informed the surgeon and recommended that the surgery be concluded early. The left radial artery was catheterized for blood sampling and blood pressure monitoring. A central venous catheter was inserted at the right internal jugular vein via blind Seldinger technique. Central venous pressure was not measured. Arterial blood gas analysis showed pH 7.12, PCO2 50 mmHg, PO2 65 mmHg, HCO3− 16.3 mM, and FiO2 1.0. Serum sodium and potassium levels were 117 mEq/L and 2.5 mEq/L, respectively. Forty minutes after the event, SpO2 was 90%, and PETCO2 was 15 mmHg.
For hysteroscopy, 6,000 ml of Urosol® solution (a mixture of 2.7% sorbitol and 0.54% mannitol) was used. At the end of the operation, the drainage quantity of the distending media comprised about 4,000 ml, but the exact amount was not confirmed. Total anesthetic time was 160 min. During the first hour, 200 ml of Ringer’s solution was infused, and urine output was 100 ml. During the second hour and the subsequent 40 min, 200 ml and 350 ml of Ringer’s solution was infused, and urine output was 1,000 ml and 600 ml without diuretics, respectively.
The patient was transferred to the intensive care unit (ICU) without tracheal extubation after chest and brain computed tomography (CT) scans. Chest CT showed pulmonary edema and acute respiratory distress syndrome (Fig. 1). Brain CT showed bilateral prominent superior ophthalmic vein dilation (Fig. 2). No intracranial hemorrhage or brain swelling occurred. CT pulmonary angiography and CT lower extremity venography was performed and showed no evidence of thromboembolism. Echocardiography performed 6 h after the event showed proper contractility and no dilation of the right ventricle but left ventricle apical ballooning, global hypokinesia of the mid to apical segments, and akinesia of the interventricular septum from the mid to apical segments (Fig. 3). Postoperative 12-lead electrocardiogram (ECG) revealed sinus tachycardia of 109 beats/min without ST-segment or T wave abnormality. Serum troponin I and CK-MB were 0.082 ng/ml and 3.0 ng/ml, respectively. Urine output was 1,780 ml without diuretics during the first five hours after entering the ICU.
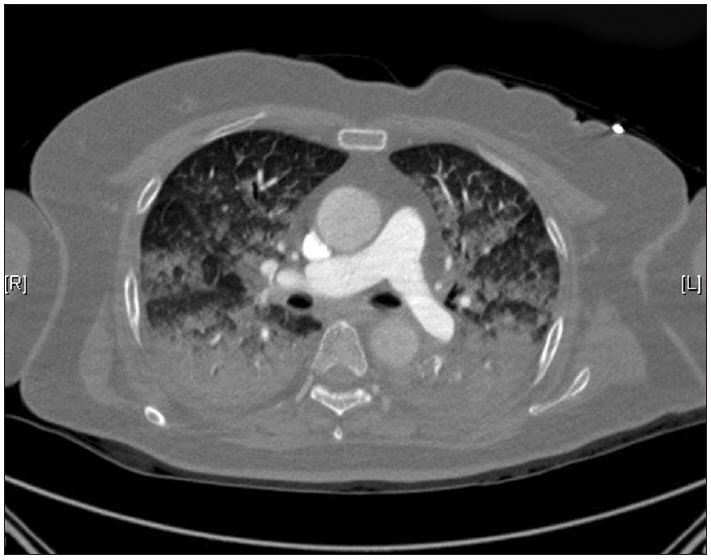
Chest computed tomography shows dependent consolidation, ground glass opacity, and interlobular septal thickening in both lungs. Probable pulmonary edema or acute respiratory distress syndrome.
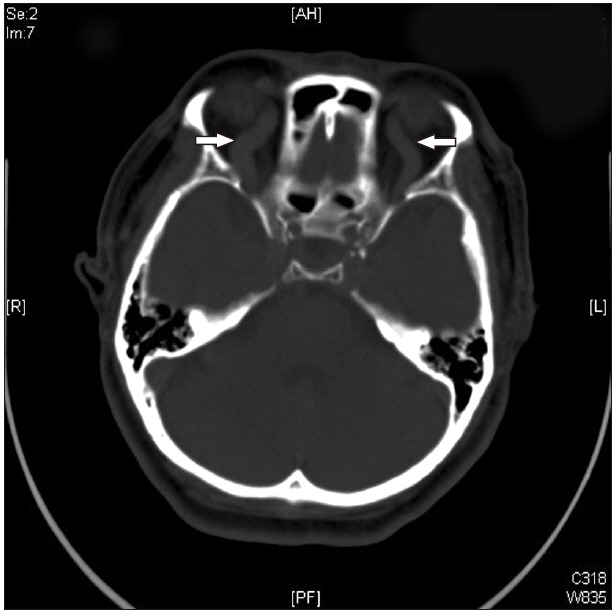
Brain computed tomography. Superior ophthalmic vein was severely dilated (arrows), and no intracranial hemorrhage was found.
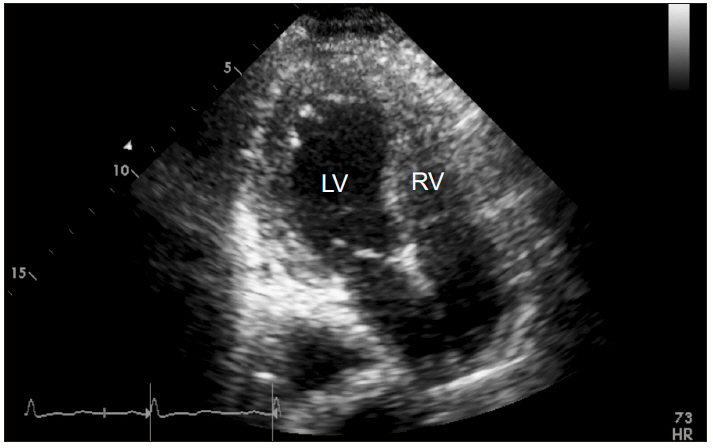
Transthoracic echocardiographic apical 4-chamber view (endsystolic phase) findings in intensive care unit after hysteroscopic surgery. Left ventriclar dilation and global hypokinesia were observed. LV: left ventricle, RV: right ventricle.
On the first postoperative day, ECG revealed sinus tachycardia (120 beats/min) with nonspecific T wave abnormality. The serum sodium level was 127 mEq/L, potassium level was 3.9 mEq/L, and cardiac biomarkers were elevated with troponin I of 9.28 ng/ml (normal ≤ 0.046 ng/ml) and CK-MB of 18.54 ng/ml (normal ≤ 3.7 ng/ml). Follow-up echocardiography showed continued regional wall motion abnormality (RWMA), but the severity was decreased. On the second postoperative day, the anteroposterior chest radiograph (chest AP) showed improved pulmonary edema. Arterial blood gas analysis showed pH 7.46, PCO2 30 mmHg, PO2 183 mmHg, HCO3− 21 mM, and FiO2 0.3. The patient’s serum sodium level was 143 mEq/L, potassium level was 3.6 mEq/L, and cardiac biomarkers returned to normal ranges. Extubation was performed, and the patient was transferred to the general ward. On the fifth postoperative day, the patient was discharged without any sequelae. At one week after discharge, echocardiography showed normal myocardial systolic function.
DISCUSSION
Complications can occur due to fluid overload during hysteroscopic surgery. Among the various reasons, direct media absorption through an exposed vessel or sinus during surgery is the main cause of fluid overload [3]. When hypotonic electrolyte-free fluid is used, hypervolemia, hyponatremia, and hypo-osmolality can occur. With Urosol® solution (a mixture of 2.7% sorbitol and 0.54% mannitol), metabolism of sorbitol may occur up to metabolic acidosis.
Severe hyponatremia can produce various neurological symptoms [4]. In this case, even in severe hyponatremia, neurological symptoms, such as nausea, headache, and seizure, were not found because the patient was under general anesthesia. Hence, fluid overload and hyponatremia went undetected until pulmonary edema developed.
We first suspected pulmonary thromboembolism and right heart failure based on the clinical features of sudden bradycardia, neck vein dilation, active nasal bleeding, and IV line regurgitation. However, CT pulmonary angiography and chest CT showed no evidence of pulmonary artery embolism, and CT lower extremity venography showed no deep vein thrombus.
According to Istre et al. [5], when 1,000 ml of fluid is absorbed, plasma sodium is reduced by 10 mEq/L. In the operating room, the plasma sodium level was 117 mEq/L. Therefore, this patient can be assumed to have absorbed 2.5 L of distending media. Consequently, it is reasonable to believe that severe water absorption caused pulmonary edema. The right heart failure thought to be accompanied by this was likely due to an increase in pulmonary vascular resistance caused by hypoxic pulmonary vascular constriction and a catecholamine surge caused by sudden pulmonary edema [6].
Urine output during the operation and the five postoperative hours was 1,700 ml and 1,780 ml, respectively. Spontaneous recovery of right ventricular function was thought to be associated with rapid recovery of intracardiac blood volume due to increased urine volume. This was confirmed by bedside echocardiography, which was performed about six hours after the event, showing both proper contractility and no dilation of the right ventricle. However, echocardiography showed left ventricle apical ballooning, global hypokinesia of the mid to apical segments, and akinesia of the interventricular septum from the mid to apical segments. Postoperative electrocardiography showed sinus tachycardia of 109 beats/min and no other abnormalities. Cardiac biomarkers were slightly elevated, with troponin I of 9.28 ng/ml (normal ≤ 0.046 ng/ml) and CK-MB of 18.54 ng/ml (normal ≤ 3.7 ng/ml).
The first postoperative day, follow-up echocardiography showed improved left ventricular RWMA. The second postoperative day, cardiac biomarkers returned to normal ranges. At one week postoperatively, echocardiography showed diminishing RWMA and normal global left ventricular systolic function. Based on the patient’s clinical features and echocardiography results, we suspect a diagnosis of Takotsubo cardiomyopathy. The pathophysiology is not well established, but catecholamines are thought to play an important role in inducing Takotsubo cardiomyopathy.
Some cases have been reported in which severe hyponatremia is associated with Takotsubo cardiomyopathy [7,8]. It is thought that excessive catecholamines are released secondary to abnormal central nervous system function. This elevated level of catecholamines has the potential to injure myocytes directly or indirectly, inducing multi-vessel coronary spasm [7]. According to Kolar et al. [9], in the hyponatremia state, intracellular calcium overload may occur due to impaired function of myocyte membrane sodium-calcium pumps.
Catecholamine level, such as those of epinephrine and norepinephrine, was not measured in this patient. Therefore, we cannot verify the specific mechanism of cardiac dysfunction in. She had no previous cardiac-related symptoms. Coronary angiography was not performed due to medical cost. Absence of coronary angiography is a major challenge in diagnosis of Takotsubo cardiomyopathy. Nevertheless, we concluded Takotsubo cardiomyopathy triggered by severe hyponatremia based on clinical features and echocardiography findings. In this case, therefore, we conclude that Takotsubo cardiomyopathy exacerbated pulmonary edema induced by volume overload and venous congestion, resulting in decreased pulmonary function and epistaxis.
In summary, hysteroscopic surgery is less complex than laparotomy. However, serious complications, such as fluid overload and cardiomyopathy, can occur during the procedure. Therefore, anesthesiologists and surgeons should pay close attention to variables such as saturation, capnography, and distending media volume during hysteroscopic surgeries.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
