Fatal pulmonary aspiration during balanced sedation with dexmedetomidine and midazolam - A case report -
Article information
Abstract
Recently, balanced sedation has commonly been used during procedural sedation. Dexmedetomidine is known for its relative safety to cause “conscious sedation” with little respiratory depression but has some limitations such as frequent awakening and hemodynamic instability during surgery. To facilitate sedation, a small dose of midazolam can be co-administered rather than escalating the dose of dexmedetomidine, especially in elderly patient. Despite the respiratory safety profile of dexmedetomidine, the overall safety of co-administration has not been clarified. We describe the first case of fatal pulmonary aspiration that developed in an elderly patient during balanced sedation with spinal anesthesia for elective femur fracture surgery.
An ideal sedative should have a fast onset and offset of drug efficacy, be predictable and titratable, and have no serious side effects. Additionally, low price burden is preferable.
Dexmedetomidine is a selective α2-adrenergic receptor agonist and is known to be a safe sedative that causes little respiratory depression [1]. It is characterized by “conscious sedation” and a clean and easy arousal from sedation, similar to being awakened by natural sleep [2]. Despite its expanded use within variable populations for procedural sedation, life-threatening pulmonary aspiration associated with dexmedetomidine has rarely been reported in cases of procedural sedation [3]. However, dexmedetomidine has some disadvantages, such as slower onset, frequent in-procedural awakenings, hemodynamic instability, and longer stay in postoperative care units [4,5]. Recently, “balanced sedation” has been introduced as an alternative strategy to increased doses of dexmedetomidine. Some sedatives or opioids such as ketamine, midazolam, or other opioids (e.g., remifentanil infusion) can be co-administered. However, the coadministration of other drugs can not guarantee the complete safety [6,7].
We describe a case of fatal pulmonary aspiration that developed in balanced sedation with dexmedetomidine and a small amount of midazolam during spinal anesthesia in an elderly patient.
CASE REPORT
The patient was an 84-year-old male (155 cm in height and 52 kg in weight) with a history of hypertension and kyphoscoliosis. He was transferred to our trauma center because of periprosthetic fracture of the left hip. He had undergone hip hemiarthroplasty 4 years ago and again fractured at the same position. He had cane-assisted ambulation prior to surgery and was relatively healthy for his age. Following routine preoperative examinations, including laboratory investigations, the patient was scheduled for elective revision hip hemiarthroplasty. He was alert and not delirious. He was not hypotensive or hypoxemic. Spinal anesthesia with adequate sedation was planned to proceed in consideration of an old age. The patient had adequately fasted from the midnight before the surgery.
In the morning on the day of surgery, the patient took his medication for hypertension. Upon arrival to the operating room, his oxygen saturation (SpO2) was 93–95%, blood pressure (BP) was 110/75 mmHg (within 20% of baseline in the ward), and body temperature was 36.9°C. Invasive arterial pressure was continuously monitored via right radial artery cannulation. Partial pressure of oxygen (PaO2) at room air was 69 mmHg. The patient was placed in the lateral decubitus position, and spinal anesthesia was performed using 12 mg of 0.5% hyperbaric bupivacaine and 20 µg of fentanyl through L4/5. The level of anesthesia was checked T6, and 6 L of oxygen was supplied via a facial mask.
Sedation was initiated with 1 µg/kg of dexmedetomidine for 10 min, followed by a 0.5-µg/kg/h infusion. A bispectral index (BIS) monitor was placed on the left forehead and monitored throughout the surgery. He fell asleep 16 min after dexmedetomidine administration. During the loading dose, his BP dropped to 78/44 mmHg, and he exhibited bradycardia of 40–44 beats per min. He was administered 5 mg of ephedrine and 100 µg of phenylephrine.
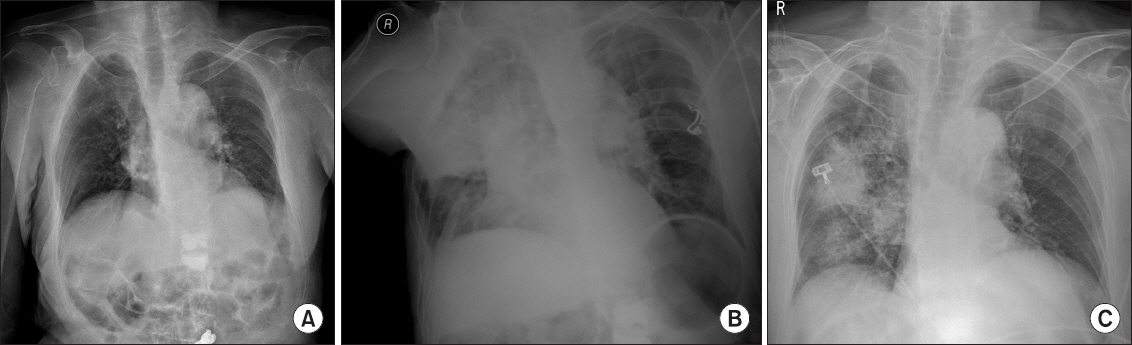
Because of frequent hammering and handling throughout the surgery, the patient started to wake up often beginning 20 min after the start of the surgery. His BIS rose to ≥80, and the dexmedetomidine dose was increased to 0.7 µg/kg/h. The surgery was highly complicated because of the revision approach. The estimated blood loss was approximately 1,200 ml. Two hours after anesthesia, the patient began to move because of uncomfortable posture and frequent awakening; however, he did not display signs of pain. Anesthetic team chose balanced sedation and concurrently administered 2 mg of midazolam, considering his hemodynamic vulnerability. The patient was finally moderately sedated and immobile. His BIS was within the range of 50–75, and self-respiration was regular. However, 15 min after midazolam administration, his SpO2 reduced to 88–92%. He did not exhibit coughing, retching, or vomiting, but there was a small amount of gastric juice in his mouth. Further, his BP decreased to 88/44 mmHg. All sedatives were stopped, and we encouraged the patient to take a breath. The patient was not dyspneic and responded appropriately to our request. His SpO2 was maintained at 93–97% with 10 L of oxygen via a facial mask. Dopamine was administered at 5–10 µg/kg/min to restore BP. Given the patient’s improper lateral positioning, the surgery was hastened. A chest X-ray in the operating room (Fig. 1) revealed diffuse infiltration, suggesting pulmonary aspiration in the right lung. For close monitoring and management, the patient was transferred to the intensive care unit (ICU) postoperatively. The duration of the surgery was 183 min, and the duration of anesthesia was 238 min.
After arrival at the ICU, the patient’s BP was 127/88 mmHg and heart rate 128 beats per min. Dopamine was administered at 10 µg/kg/min, and oxygen was supplied via a 6-L facial mask. Arterial blood gas analysis revealed an SpO2 of 97%, PH of 7.39, PaO2 of 59 mmHg, partial pressure of CO2 of 41 mmHg, and a base excess of −0.2 mM. The patient could say his name but could not correctly respond when asked about the time and place.
Antibiotics were administered, and conservative lung treatment was given to the patient. In ICU, his BP gradually decreased and SpO2 did not improve. The patient became irritable and dyspneic. Norepinephrine was administered, and endotracheal intubation was performed.
There was no significant difference in chest X-ray findings, and his body temperature was 37.5–38.0°C. In the night on the surgery day, there was an episode of paroxysmal supraventricular tachycardia, and 6 mg of adenosine was administered. His BP remained low, between 75 and 90 mmHg, despite the administration of 20 µg/kg/min of dopamine and 0.3–6 µg/kg/min of norepinephrine. The gastric acid-tinged copious secretions persisted in endotracheal suction, and PaO2 was maintained within 50–70 mmHg.
Despite the use of maximal doses of inotropes and supportive care, multiorgan failure progressed rapidly. He showed the signs of severe metabolic acidosis, increased creatinine levels, markedly increased hepatic enzyme levels, and persistent hypotension. The patient died on the night after the surgery.
DISCUSSION
We report a case of fatal pulmonary aspiration in balanced sedation with dexmedetomidine and a small amount of midazolam during spinal anesthesia of an elderly patient with a femur fracture undergoing revision hip hemiarthroplasty.
Although the postoperative outcomes associated with anesthetic methods in elderly patients undergoing hip fracture surgeries are a subject of ongoing debate, regional anesthesia is often preferred for its association with reduced postoperative complications and shortened hospital stays [8]. Elderly patients may not remain immobile long enough to allow a surgery to be completed; consequently, moderate to deep sedation is often required. The potential complications of deep sedation include hypoventilation, apnea, airway obstruction, aspiration, and hemodynamic instability [9]. For elderly patients, safer sedation strategies with lesser respiratory depression and stable hemodynamics are required [10].
We selected balanced sedation using a dexmedetomidine infusion with intermittent midazolam. Dexmedetomidine is a relatively new drug that has been widely used for procedural sedation. It is a selective α2-adrenergic receptor agonist (α2: α1 ratio of 1,620:1) that induces sedation and anxiolysis in a dose-dependent manner. Because central α1-adrenoceptor activation offsets the sedative α2-adrenergic effects, greater selectivity leads to greater sedation potency of the effect of the α2 receptor [11]. Dexmedetomidine has a unique sedation effect, similar to natural sleep, because of its effects on central pre- and postsynaptic α2 receptors in the locus coeruleus rather than through gamma-aminobutyric acid (GABA) receptors as with other drugs, such as propofol or midazolam [12]. This permits the patient to easily awaken to a lucid state and cooperate with instructions, referred to as “conscious sedation.” [11].
Since first developed for use in patients receiving mechanical ventilation in 1999, the use of dexmedetomidine has been widely expanded to general anesthesia, postoperative sedation, and procedural sedation. Moreover, there have been various off-label applications ranging from pediatric to elderly patients, intranasal or buccal administration, co-administration of an adjuvant to local analgesia techniques, and balanced sedation with other sedatives or opioids [11]. Despite its respiratory safety, its safety in off-label use with elderly patients and co-administration with other medications has not been clarified [11].
The incidence of pulmonary aspiration during sedation is extremely rare and much lower than that of respiratory complications [3]. Pulmonary aspiration occurs when there is a loss of airway reflexes and elevated pressure in the stomach, sufficient to overcome the lower esophageal sphincter tone. Although several drugs, such as propofol and opioids, can reduce the lower esophageal sphincter tone [13], it is difficult to elevate the intragastric pressure to overcome the lower esophageal sphincter tone. This is because the stomach is very distensible and there is a very large reserve of volume till the intragastric pressure increases. In the usual setting of anesthesia or sedation without intestinal obstruction or insufficient mask ventilation, the development of significant pulmonary aspiration is uncommon [13].
Most cases of pulmonary aspiration reportedly develop during airway manipulation in the general anesthesia. Even in the procedural sedation, many cases of aspiration have been reported in the endoscopic procedure [3]. In non-endoscopic procedures, the incidence of aspiration was low and most patients recovered well. Only one case of non-endoscopic procedural sedation in an immunocompromised patient resulted in death [14]. Moreover, there has been no report of death associated with pulmonary aspiration with dexmedetomidine sedation [3].
In the present case, the patient maintained “conscious sedation” throughout the procedure; however, the addition of a small dose of midazolam to the dexmedetomidine infusion allowed the patient to attain a transient deep sedation state. Consequently, aspiration was possible due to the weakening of the protective airway reflex because of synergic effects on pharyngeal function [7]. Although the aspirated volume was small, it was more acidic than larger volumes and caused more severe and irreversible pulmonary sequelae [15].
Except old age and the use of sedatives, there were no such specific predisposing factors for pulmonary aspiration in this case. The patient had a full fasting time and no gastric obstruction, abnormal reflux, or stasis [3].
Considering the rapidly progressive clinical course, other possible causes that required differentiation included myocardial ischemia, stress-induced cardiomyopathy, or pulmonary embolism. However, the patient’s risk of pulmonary embolism was small given that he only underwent 2 days of immobilization. Moreover, there were no apparent ischemic findings on electrocardiogram (EKG) or symptoms of chest pain. Further evaluations, such as computed tomography scans, were not performed given the patient’s unstable hemodynamics.
Incidental pulmonary aspiration resulted in acute respiratory distress syndrome and pulmonary hypertension. Subsequent systemic hypoperfusion, severe metabolic acidosis, and multiorgan failure drove the patient into sudden collapse. The difficulties in maintaining oxygenation and perfusion pressure appear to have accelerated this deterioration. Applying extracorporeal membrane oxygenation (ECMO) may have been helpful but was not implemented because of family refusal [13].
This case is the first report of fatal pulmonary aspiration that developed in balanced sedation with dexmedetomidine and low-dose midazolam during spinal anesthesia. Keeping aside the perceived safety of the medication used and the duration of the presurgical fast, any procedural sedation with the possibility of losing consciousness can induce severe pulmonary aspiration. Care should be taken to select a sedation method that completely considers both risks and benefits. Furthermore, meticulous dose selection and the ability to immediately respond to adverse effects are essential, particularly in elderly or compromised patients.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
ORCID
Jeong Heon Park: https://orcid.org/0000-0003-3852-5390
Sang Yun Kim: https://orcid.org/0000-0001-8764-9942