Morphologic changes in the spinal cord following intrathecal palonosetron-HCl injection in rats
Article information
Abstract
Background:
Intravenous palonosetron-HCl, a second-generation antagonist of selective serotonin type 3 (5-HT3) receptors, can prevent chemotherapy-induced nausea and vomiting (CINV) and postoperative nausea and vomiting (PONV). 5-HT3 receptors are abundant in the lower brainstem and the substantia gelatinosa of the spinal cord, which provides a theoretical rationale for neuraxial administration of 5-HT3 receptor antagonists for CINV, PONV, and opioid-induced nausea and vomiting. However, there are no reports of neuraxial administration of palonosetron-HCl. Before neuraxial administration of a drug is accepted for clinical use, its safety must be proven. This study was conducted to determine whether neuraxial administration of palonosetron-HCl produces neurologic injury.
Methods:
Male Sprague-Dawley rats under general anesthesia were catheterized intrathecally and the catheter tip was advanced caudally to the L1 vertebra. After 7 days, 20 μl of normal saline (N group, n = 6) or 20 μl (1 μg) of palonosetron-HCl (P group, n = 6) were injected intrathecally once per day for 2 weeks. Neurotoxic changes were evaluated by light microscopy (LM) and electron microscopy (EM) of the spinal cord. Behavioral changes were also evaluated in both groups.
Results:
One of the N group rats and three of the P group rats demonstrated abnormal behavior during intrathecal drug injection, but otherwise their behavior was normal. The spinal cords of the N group did not have any abnormal findings by LM or EM. The spinal cords of the P group had multiple vacuoles in the white matter by LM, especially in the dorsal funiculus, and EM revealed myelin, axonal, and mitochondrial swelling.
Conclusions:
Results suggest that chronic intrathecal administration of palonosetron-HCl produced microscopic morphologic changes in the spinal cords of rats.
INTRODUCTION
Neuraxial drug administration is a drug injection via an epidural or intrathecal route. Neuraxial administration is used for a variety of drugs to treat acute or chronic pain as well as spastic disorders [1]. Palonosetron-HCl (Aloxi, Pierre-Fabre Medicament Production Aquitaine Pharm International, France) is a second-generation serotonin type 3 (5-HT3) receptor antagonist indicated for the prevention of chemotherapy-induced nausea and vomiting (CIVN) and post-operative nausea and vomiting (PONV) [2]. Ondansetron is a first-generation 5-HT3 receptor antagonist widely used to prevent nausea and vomiting during radiation therapy and chemotherapy [3,4] and is effective for nausea and vomiting induced by opioid administration after surgery. In addition, ondansetron does not have a neurotoxic effect following intrathecal administration in rats, and epidural administration is more effective than intravenous injection for the prevention of postoperative nausea and pruritus after cesarean section [5]. Palonosetron-HCl is expected to prevent nausea and vomiting after the administration of opioids due to its inhibitory effect on 5-HT3 receptors in the central nervous system during neuraxial opioid administration. There are few reports on the role of palonosetron-HCl in the prevention of opioid induced nausea and vomiting [6], but no reports are available on the neuraxial administration of palonosetron-HCl.
We designed this study for two scientific and clinical reasons. First is whether Palonosetron-HCl is safe as ondansetron via intrathecal route, thus we can use it for epidural patient controlled analgesia. Second is to provide evidence or reference when palonisetron-HCl is injected to neuroaxial space inadvertently.
To prevent neurotoxicity and confirm the safety of treatments before clinical application in human, epidural and spinal injection must be performed in animal models to examine histological, physiological, and behavioral changes. This study was designed to determine the safety of long-term intrathecal palonosetron-HCl administration in rats.
MATERIALS AND METHODS
The study was approved by the Uijeongbu St. Mary Hospital Institutional Animal Care and Use Committee. Twelve male Sprague–Dawley rats weighing 250–300 g were used. Rats were housed individually at the experimental facility for 1 week prior to the experiment. Rooms were maintained under a 12/12 h light-dark cycle at a temperature of 22°C. Rats were allowed free access to food and water.
Surgical procedure for intrathecal catheterization
Rats were anesthetized with 5% isoflurane in oxygen, and the subarachnoid space was cannulated with a polyethylene tube (PE-10, Becton Dickinson, USA) through the atlanto-occipital membrane using the modified method of Yaksh and Rudy [7]. The catheter tip was advanced 8 cm caudally to the L1 vertebra. The other end of the catheter was affixed to the upper back and sealed with a stainless-steel plug. The wound was closed, and rats were allowed to recover for 7 days. Rats with symptoms of traumatic nerve damage during the 7-day recovery period were excluded from the study.
Intrathecal drug administration
Seven days after the intrathecal catheterization procedure, rats were randomized into two groups. In the P group (n = 6), palonosetron-HCl (Aloxi, Pierre-Fabre Medicament Production Aquitaine Pharm International, France) was administrated intrathecally in a volume of 20 μl (1 μg) followed by a 10-μl normal saline catheter flush. In the N group (n = 6), animals were injected with 20 μl of isotonic saline followed by a 10-μl saline catheter flush. Injections were repeated every 24 h for 14 days and rats were observed for any signs of neurologic impairment. Drug administration was completed over 1 min using a Hamilton syringe (Hamilton microliter syringe, Hamilton Co., USA) under isoflurane anesthesia.
Sample preparation
Twenty-four hours after the last intrathecal injection, rats were anesthetized with isoflurane and perfused with 400 ml of normal saline and 400 ml of 4% paraformaldehyde. The entire spinal cord was removed with the catheter following bilateral laminectomy of the spine. The L1 segment was cut with a vibratome and fixed in 4% paraformaldehyde and 2.5% glutaraldehyde. Fine sections (1 μm) were stained with toluidine blue for light microscopy. Ultrathin sections (500 Å) were stained with uranyl acetate and lead citrate for electron microscopy. The histological examination was performed in all rats of each group by a pathologist.
RESULTS
Rats ate and drank normally during the experimental period and each group had similar increases in weight per day (2–6 g/day). Rats in both groups demonstrated no neurological abnormalities during catheter insertion and the 7-day follow-up period. One N group rat had a breath-holding episode during the third infusion, but recovered after injection.
Three rats in the P group had behavioral responses indicating pain during the intrathecal infusion. The first of these three P group rats developed severe pain in the left hind foot after the first infusion and was euthanized. The necropsy revealed that the catheter was placed in the anterior portion of the spinal cord at the L1 level. No bleeding or tissue necrosis was observed. The second P group rat stretched the left hind foot during the third injection and maintained a regular tremor for 2–3 min, but normal behavior returned after emergence from anesthesia. The rat repeated these signs during each subsequent drug injection. The third rat extended its tail during the eleventh infusion, but displayed normal behavior after arousal from anesthesia. In neurologic examination, there is no abnormal impairment in all groups.
No gross bleeding or necrosis of the spinal cord was observed in animals with either normal or abnormal posture during drug infusion. Light microscopy of the N-group spinal cords revealed a normal vascular distribution in the white and gray matter. Neurons and the nuclei of astrocytes (glial cells) in the gray matter of the N group were normal, and no unusual pathologic findings were observed (Figs. 1A and 1B). In contrast, the P group developed many vacuoles in the white matter, particularly in the dorsal funiculus (Fig. 1C). The white and gray matter showed normal vascular distribution. The nuclei of motor and glial cells were normal, and no infiltration of inflammatory cells was observed. The electron microscopy findings of the P group included separation of the myelin inner lamellar structure and swelling and degeneration of myelin creating fluid-filled spaces (Figs. 2B and 2C). This was observed in a swollen axon wrapped with thinner myelin, and enlarged mitochondria and degeneration of the mitochondrial cristae in the axon were also observed (Fig. 2D). In addition, swelling of the mitochondria was observed in axons in white matter regions without vacuoles (Fig. 2D). A yellowish, mucus-like material was observed with the naked eye around the intrathecal catheter, and fibrosis and infiltration of macrophages and neutrophils were observed by light microscopy in both group (Fig. 3).
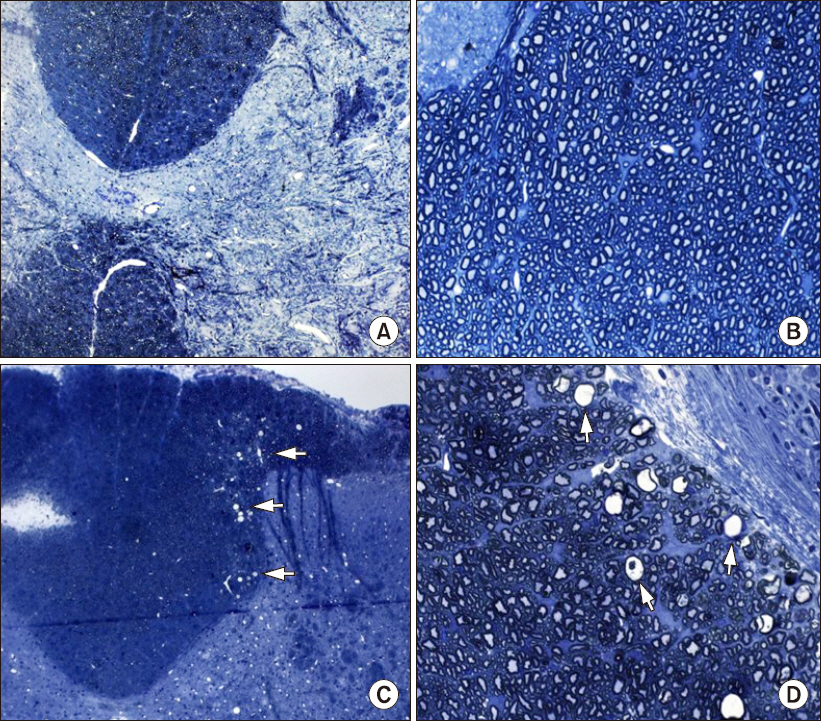
Light microscopy of rat spinal cords stained with toluidine blue. (A, B) Spinal cord of a administered rat normal saline (A: 100×, B: 400×). (C, D) Spinal cord of a rat administered palonosetron-HCl (C: 100×, D: 400×). A and C demonstrate no identifiable hemorrhage and no necrosis, but C has many vacuoles (arrows) in the dorsal funiculus. B has normal myelin and axons. D has thinly myelinated, swollen, and degenerating axons (arrows).

Electron microscopy of rat spinal cords. The spinal cord of a rat administered normal saline (A) and a rat administered palonosetron-HCl (B–D). (A) Compact and myelinated axons. (B) Myelinated axons with separation of the inner lamellae (arrow) creating fluid-filled spaces within the myelin. Axons surrounded by glial cell (arrow head). (C) Thinly myelinated spinal cord with swollen axons (arrow head) and intramyelinic fluid accumulation (arrow). (D) Hypertrophied and degenerated mitochondria (arrow) in the thinly myelinated axon. Black bars represent 1 μm in A and C, 2 μm in B, and 0.5 μm in D.

Light and electron microscopy of the intrathecal catheter in both groups. (A) Light microscopy (40×) of the intrathecal catheter (arrow). (B) and (C) Electron microscopy of tissue surrounding the intrathecal catheter. A macrophage (arrow) can be seen in B, and neutrophils in C (arrows). Black bars in B and C represent 2 μm.
DISCUSSION
Palonosetron-HCl (Aloxi, Pierre-Fabre Medicament Production Aquitaine Pharm International) is a second-generation 5-HT3 receptor antagonist with a high affinity for the 5-HT3 receptor and a longer plasma half-life than first-generation 5-HT3 receptor antagonists such as ondansetron or granisetron [8]. Ondansetron and granisetron are competitive antagonists that compete directly with serotonin at the receptor-binding site, but palonosetron-HCl binds allosterically (i.e., binding at a different site compared to serotonin) and creates a conformational change in the serotonin receptor that inhibits serotonin binding. Palonosetron-HCl has a long-term inhibitory effect on 5-HT3 receptor function by inhibiting serotonin-dependent Ca2+ influx in vitro [9]. Therefore, palonosetron-HCl is more effective than first-generation 5-HT3 receptor antagonists in treating both delayed and overall nausea and vomiting after chemotherapy [10,11].
Neurotoxicity is not observed when ondansetron is administered to rats intrathecally [5]. Intrathecal administration of ondansetron prevents postoperative nausea and pruritus after cesarean section more effectively than intravenous administration [5]. A single bolus injection of intrathecal ondansetron dose in rabbits did not produce clinical or histological neurotoxicity, and intrathecal administration can also provide pharmacokinetic advantages over systemic administration of ondansetron [12]. 5-HT3 receptors exist in the central nervous system of many species, including humans, and are particularly abundant in the lower brainstem and the substantia gelatinosa of the spinal cord [13,14], which is the theoretical base for intrathecal 5-HT3 antagonist administration. Despite the wide use of palonosetron-HCl, intrathecal administration has not been reported. Thus, animal spinal experiments were conducted in this study to ensure the safety of intrathecal palonosetron-HCl in clinical applications.
In this study myelin, axonal and mitochondrial edema and degradation were observed in the P group, but not the N group. The P group also had many vacuoles in the dorsal funiculus of the white matter. One rat in the N group and three in the P group had behavioral responses indicative of pain during intrathecal palonosetron-HCl infusion. The abnormal behavior observed during intrathecal infusion in these rats was caused by temporarily elevated pressure during the infusion because neovascularization and ischemic changes were absent in the spinal cord and rats demonstrated normal behavior after injection. No significant microscopic differences were observed in the spinal cords of rats that had adverse reactions during infusion compared to rats that had normal reactions.
Vranken et al. [15] evaluated neurotoxicity during continuous intrathecal administration of S (+)-ketamine alone or combinations of S (+)-ketamine, morphine, bupivacaine, and clonidine in a patient suffering from intractable neuropathic cancer pain. Postmortem observation of the spinal cord and nerve roots revealed severe histological abnormalities including central chromatolysis, nerve cell shrinkage, neuronophagia, microglial up-regulation, and gliosis without the presence of abnormal clinical behavior or neurologic symptoms [15]. Also, the entire cord had extensive subpial vacuolation and an almost circular loss of myelin staining. Loss of myelin was also noted in the dorsal horn of the spinal cord and was more pronounced in the region around the tip of the catheter [15]. In this study, many vacuoles were observed in the white matter, particularly in the dorsal funiculus around the catheter tip, under light microscopy in the P group. The electron microscopy findings of the P group also included the separated structure, swelling, and degeneration of myelin. Peripheral vacuolation of the spinal cord is a nonspecific finding [16]. Peripheral vacuolation is observed during human autopsy without any prior clinical signs and is generally regarded as a clinically insignificant artifact. However, this phenomenon does indicate drug neurotoxicity in the presence of macrophages and microglial up-regulation [15]. In another study, characteristic histopathological changes in neurotoxicity included vacuolization of the dorsal funiculus and chromatolytic changes in motor neurons [17]. There is a close correlation between the degree of vacuolation of the dorsal funiculus and paresthesia [18].
We were convinced that these changes were not indicative of palonosetron-HCl neurotoxicity but nonspecific toxicity, because there were no macrophages, microglial up-regulation, or motor neuron chromatolytic changes while microscopy demonstrated myelin and axonal swelling. Also, none of the rats in the P group demonstrated abnormal behavior.
As the other reason, the microscopic findings of both groups suggested an inflammatory response around the catheter, and the presence of the catheter alone could cause infiltration of lymphocytes and fibrosis [19]. However, only the P group developed multiple vacuoles in the white matter, particularly in the dorsal funiculus. This vacuolation occurred in the absence of neovascularization, which is often present during infection. Therefore we concluded that the myelin, axonal, and mitochondrial edema and degradation observed in the P group were not caused by infection.
If the histological changes and lesions observed in this study were expanding or the body lost its ability to compensate, neurologic symptoms could be expressed. Morphologic results are important and should not be overlooked because functional changes follow morphologic changes [20]. Since morphologic changes were observed in the spinal cords of palonosetron-HCl injected rats, additional studies using a larger animal species should be conducted.
Further study is also needed to determine the relationship between the dose of palonosetron-HCl and neurotoxicity. Yamashita et al. [17] reported that high dosages and concentrations of local anesthetics in rabbits could cause neurotoxicity. In the present study, 1 μg (20 μl) dose of palonosetron-HCl was injected into rats intrathecally. This dose is equivalent to the 0.25 mg dose used to prevent CINV in adults, and 2.7 fold more than the 0.075 mg dose used to prevent PONV (assuming the average rat weighs 300 g). In addition, the difference between intravenous and intrathecal dose efficacy was not considered. Thus it is possible that a relatively high dose of palonosetron-HCl could produce histological changes.
The limitations of this study were as follows. First, paresthesia—which is closely related to the vacuolation of the dorsal funiculus—was not evaluated. Second, this study had a small sample size and used a small experimental animal species. Third, neurotoxicity was evaluated at a fixed dose interval. Research is needed to determine if neurotoxicity occurs with variable doses and/or with variable durations. Fourth, future research should determine if drug-induced neurotoxicity, when it occurs, is permanent or reversible.
In summary, palonosetron-HCl was administered to rats intrathecally for 2 weeks without the development of neurologic or behavioral symptoms. However, morphologic changes, such as vacuolation of the dorsal funiculus and myelin, axonal and mitochondrial swelling and degeneration were observed by electron microscopy even if these changes were regarded as nonspecific toxicity.