Ventilation through a straw
Article information
Abstract
Transtracheal jet ventilation can be used for resuscitation of partial airway obstruction. A prerequisite for jet ventilation is that at least a minimum airway opening for gas escape must be secured. Therefore, another option should be considered in cases of complete airway obstruction. The following methods or devices has been used under cricothyrotomy using an intravenous cannula: 1) Ambu (bag valve mask) bagging, 2) Ventrain®, 3) Rapid-O2 oxygen insufflation device (Rapid-O2), and 4) jet ventilation using a dual lumen catheter. During Ambu bagging, extraordinarily high insufflation pressure is required to force oxygen through the cannula. When using a 12-G cannula, long and slow positive-pressure ventilations (10–12 breaths/min) are required, which makes it extremely difficult to compress the bag. Therefore, a 10-G or larger is recommended. Ventrain® is an expiratory assist device capable of forcibly expelling insufflated oxygen through a transtracheal cannula. It is recommended to adjust the inspiratory and expiratory times while observing the chest wall movements. Rapid-O2 is a rescue oxygenation device with adequate ventilation of less importance; therefore, the resulting hypercarbia is inevitable. A 14-G cannula is used. Lastly, jet ventilation using a dual-lumen catheter with a 16-G inflow lumen and 10-G outflow lumen was used to obtain both oxygenation and ventilation. However, the addition of the outer diameters of 16-G and 10-G results in an outer diameter of 5.1 mm, which is too large to puncture the cricothyroid membrane. In conclusion, Ventrain® is considered the most ideal device for use among the devices developed to date.
INTRODUCTION
Transtracheal jet ventilation can be used as a resuscitation method when dyspnea accompanied by hypoxia occurs due to partial airway obstruction. However, in cases of near-complete or complete upper airway obstruction, egress of the injected oxygen volume can hardly be achieved through the upper airway. In such cases, continuous application of jet ventilation may result in barotrauma to the lung, which can lead to detrimental outcomes. Therefore, a prerequisite for jet ventilation is that the minimum airway opening for gas escape must be secured. If partial, but severe, airway obstruction is present, a considerable time is required for egress of the injected volume, which is associated with a low respiratory rate, resulting in insufficient minute volume and concurrent hypercarbia. Although it has been reported that an healthy cardiovascular system can tolerate profound respiratory acidosis for prolonged periods of time [1,2], in such cases, anesthesiologists hesitate to continue jet ventilation. In short, the greater the expiratory obstruction, the lower is the need for jet ventilation. In conclusion, another option should be considered for near-complete or complete airway obstructions. Tracheostomy and cricothyrotomy using a scalpel [3] can be attempted, but the following methods under cricothyrotomy using an intravenous cannula, familiar to anesthesiologists and more easily accessible, can be attempted. In the following description, complete airway obstruction broadly includes near-complete airway obstruction.
1) Ambu (bag valve mask) bagging
2) Use of Ventrain® (expiratory assist device)
3) Use of Rapid-O2 oxygen insufflation device
4) Jet ventilation using dual lumen catheter (inflow lumen and outflow lumen)
AMBU (BAG VALVE MASK) BAGGING
Ambu bagging can be attempted by placing an intravenous cannula into the trachea through a percutaneous cricothyroid membrane puncture. The intravenous cannula is connected to the Ambu bag as follows (Fig. 1): after connecting the 8.0-mm endotracheal tube adapter to the rear side of the 3-ml syringe with the plunger removed, the Ambu bag is connected to the rear side of the 8.0-mm endotracheal tube adapter. Finally, the tip of the syringe is connected to the cannula hub. As the opening of the tip of the 3-ml syringe has an inner diameter (ID) of 2 mm, the opening must be widened to at least 2.6 mm while using the 10-G cannula (ID: 2.61 mm).
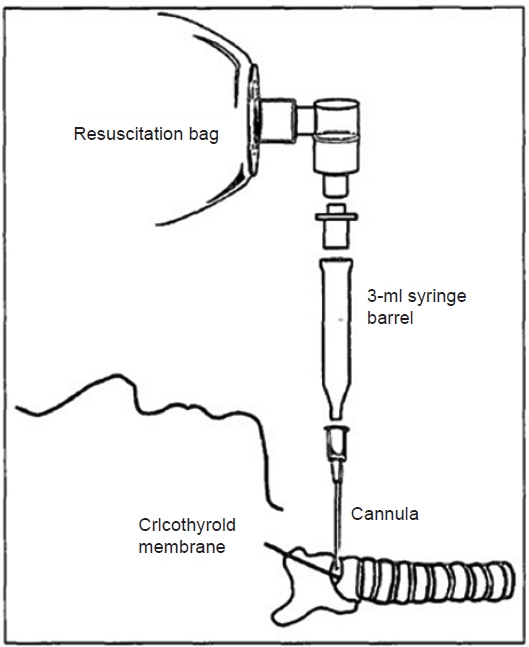
Ambu bag and connection of the the syringe. After connecting the 8.0-mm endotracheal tube adapter to the rear side of the 3-ml syringe with the plunger removed, the Ambu bag is connected to the rear side of the 8.0-mm endotracheal tube adapter. Finally, the tip of the syringe is connected to the cannula hub.
In cases of complete airway obstruction, tidal volumes of 150, 205, and 480 ml/s can be delivered with Ambu bagging using the 14-, 12-, and 10-G cannulas, respectively [4]. Regardless of the size of the cannula used, Ambu bagging provided immediate improvement in blood oxygenation. However, if a cannula with an ID smaller than 10-G (ID: 2.61 mm) is used, inadequate ventilation may occur [5]. In the case of complete airway obstruction, up to 150 ml/s through a 14-G (ID: 1.55 mm) is difficult to achieve sufficient tidal volume, and it is also very difficult to manually compress the Ambu bag to deliver the insufflating volume through a 14-G cannula, resulting in severe hypercapnia due to hypoventilation. Therefore, a 14-G cannula is not recommended [6].
Coté et al. [7] induced hypoxia by obstructing the upper airway completely in 25-kg dogs that had undergone muscle relaxation after anesthesia. Then, a percutaneous cricothyroid membrane puncture was performed with a 12-G cannula, and Ambu bagging was started with 10–12 breaths per minute with a 10 L/min oxygen supply. Immediate improvement in blood oxygenation was obtained, but during 30 min of bagging, blood carbon dioxide increased by two-fold (~60 mmHg) of the control level. During Ambu bagging, an extraordinarily high inflation pressure was required to force oxygen through the intravenous cannula into the trachea. Thus, long and slow positive-pressure ventilations (10–12 breaths/min) were required, which were extremely tiresome for the anesthesiologist and resulted in frequent changes in personnel to compress the bag. Therefore, Ambu bagging using a 14-G or 12-G cannula is not recommended, but a 10-G (ID: 2.61 mm) or larger cannula is recommended [8]. To assess the difficulty of Ambu bagging for each cannula size, we tried Ambu bagging with hands, which demonstrated that compression of the Ambu bag was very difficult with a 12-G cannula, but relatively easier, but still hard, with a 10-G cannula. Ambu bagging with a 3-mm ID endotracheal tube was slightly easier than with a 10-G cannula, and notably, there was little difficulty in compressing the bag when a 4-mm ID endotracheal tube was used. Neff et al. [5] conducted an experiment using 50-kg sheep, maintaining spontaneous ventilation after anesthesia, artificially created complete airway obstruction, and administered room air or 100% oxygen at 15 L/min through a 2.0-mm ID cannula (reference comparison: ID of 12-G cannula = 2.16 mm) mounted into the trachea. The results showed that blood oxygenation and ventilation were unsatisfactory in both the spontaneous ventilation group and the Ambu-assisted group under 100% oxygen supply. However, when a 2.5-mm ID cannula (reference comparison: ID of 10-G = 2.61 mm) was used, adequate blood oxygenation and ventilation were maintained in both groups under a 100% oxygen supply. In contrast, when a 3-mm ID cannula (reference comparison: 9-G = 2.98 mm) was used, normal blood oxygen and carbon dioxide levels were maintained in the spontaneous ventilation group under a room air supply. These results support the rationale for using a 10-G or a larger cannula to maintain the adequacy of ventilation during Ambu bagging in complete airway obstruction.
VENTRAIN®
Ventrain® (Fig. 2) is an expiratory assist device capable of forcibly expelling insufflated oxygen and can be used for complete airway obstruction [9,10]. It is recommended to use a 2-mm ID and 75-mm long transtracheal catheter (Cook Medical, USA). After connecting a wall-mounted pressure-compensated oxygen flowmeter, 15 L/min of oxygen was supplied through a Ventrain® for 2 s, followed by gas egression for 2 s.
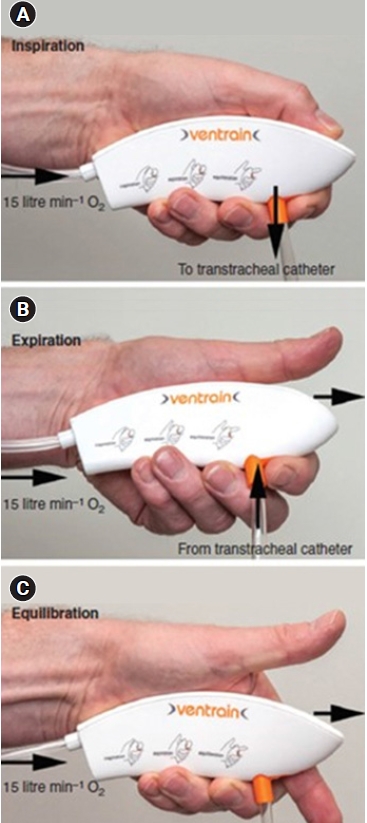
Ventrain® and its use. (A) Insufflation. (B) Expiration. (C) Equilibrium. When both apertures are closed (bypass closed and thumb closed) (A), insufflation is achieved, and when the aperture at the top (aperture of the exhaust pipe) is opened while the bottom aperture is closed (bypass closed and thumb open) (B), expiration occurs. Equilibration can be accomplished by opening both holes simultaneously (bypass open and thumb open) (C).
There are apertures at the top and bottom of the device. When both apertures are closed (bypass closed and thumb closed) (Fig. 2A), insufflation is achieved, and when the aperture at the top (aperture of the exhaust pipe) is opened while the bottom aperture is closed (bypass closed and thumb open) (Fig. 2B), expiration occurs. When the aperture at the top was opened (bypass closed and thumb open), the high-speed oxygen flow created a subatmospheric pressure and entrained gas from the transtracheal catheter by negative pressure created by the Bernoulli principle, thereby facilitating the egress of gas through the small-bore cannula. Closing both the bypass and the aperture of the exhaust pipe (bypass closed and thumb closed) redirects the flow to the side port (i.e., to the transtracheal cannula), and oxygen is insufflated. By alternatively occluding and releasing the aperture of the exhaust pipe while keeping the bypass closed, either oxygen is insufflated or subatmospheric pressure is created to assist the egress of gas through the small-bore cannula. Equilibration can be accomplished by opening both holes simultaneously (bypass open and thumb open) (Fig. 2C). At equilibrium, the lungs remains stationary. In this state, the device is claimed to be functionally switched off, with no clinically relevant flow to and from the patient. However, in reality, a small amount of exhalation occurs. Through the transtracheal cannula, approximately 250 ml/s of oxygen at a flow rate of 15 L/min was injected at a pressure of 113 cmH2O, and the forced exhalation rate from the lungs was approximately 213 ml/s at a pressure of –97 cmH2O. At equilibrium, a pressure of –6.3 cmH2O was created; therefore, even in the equilibration state, approximately 30 ml/s of exhalation occurs [11]. Air trapping may occur because of a mismatch between inspiratory and expiratory times. Conversely, if more gas is suctioned out of the patient than has been insufflated, negative-pressure pulmonary edema may develop even after a short period of high subatmospheric intrapulmonary pressure. Therefore, it is recommended to additionally adjust the inspiratory and expiratory times with observing the rise and fall of the chest wall [9,10].
Hamaekers et al. [12] attempted to ventilate through a Ventrain® at an oxygen flow rate of 15 L/min with an inspiratory to expiratory time of 1 s:1 s in pigs with non-obstructed, completely, or near-completely obstructed airways. Ventrain® immediately improved blood oxygenation and maintained normal carbon dioxide level in cases of complete or near-complete airway obstruction. However, in the absence of airway obstruction, restoration of blood oxygenation to baseline values was delayed, and blood carbon dioxide levels did not return to normal due to insufficient ventilation, proving Ventrain® to be an effective device for use in near-complete or complete airway obstruction. Several cases of the successful use of Ventrain® in near-complete obstruction in adults and children have been reported [13–15].
RAPID-O2 OXYGEN INSUFFLATION DEVICE (RAPID-O2)
RAPID-O2 [16] (Fig. 3) is a rescue oxygenation device for use in “cannot intubate, cannot oxygenate” events, or in the case of complete upper airway obstruction, which consists of a T-piece with an extension tubing attached to a cannula with a Luer lock connector. The T-piece consisted of an oxygen flowmeter connection part and a single large opening that could be closed and opened with a thumb. Thumb occlusion of the T-piece allows oxygen to flow from the flowmeter to the cannula, while releasing the thumb allows for both passively expired gas and flowmeter gas to vent into the atmosphere. In contrast to Ventrain®, a non-pressure-compensated flowmeter can be used.

Rapid-O2 oxygen insufflation device (A). (B) Inspiration. (C) Expiration. It consists of a T-piece (T-connecter) with an extension tubing attached to a cannula with a Luer lock connector. (A) The T-piece consists of an oxygen flowmeter connection part and a single large opening that could be closed and opened with a thumb. (B) Thumb occlusion of the T-piece allows oxygen to flow from the flowmeter to the cannula, while releasing the thumb (C) allows for both passively expired gas and flowmeter gas to vent into the atmosphere.
Data from animal wet lab studies by Heard [17] indicated that, by extrapolation to humans, 1,000 ml of oxygen is needed for the initial rescue of an adult, which, at 15 L/min, equates to 4 s of oxygenation. An additional 500 ml of oxygen was adequate once SaO2 began to drop after the initial oxygen supply. The rationale for using the oxygenation strategy advocated by Heard [17] is to avoid over-insufflation by providing re-insufflation only when oxygen saturation drops. A 14-G cannula was used for this purpose [18].
Wexler et al. [19], in a patient with a large base of tongue cancer causing near-complete airway occlusion, applied transtracheal oxygenation using a Rapid-O2 as a bridge to safe transtracheal jet ventilation. As jet ventilation could not be performed on an adult male patient with near-complete airway obstruction due to a large base of tongue cancer, Wexler et al. [19] attempted to remove the mass to some extent while supplying oxygen through Rapid-O2 before creating enough space for applying jet ventilation. In the awake state, a 6-Fr (= ID 2 mm) Cook Transtracheal catheter (Cook Medical) was inserted through the cricothyroid membrane. When the patient was anesthetized and SaO2 decreased to 93%, oxygen was injected using Rapid-O2. After 6–8 min, when SaO2 decreased to 93%, oxygen was injected again through the Rapid-O2. This process was repeated for 60 min until the mass was removed to some extent. A single oxygen injection was performed in the same manner as described above. Subsequently, jet ventilation was performed, and EtCO2 measured while changing to jet ventilation was 115 mmHg, showing severe hypercapnia. Although this case demonstrated the practical utility of Rapid-O2 for rescue oxygenation in near-complete airway obstruction, normal minute ventilation was barely achievable with this application strategy. For a short period of application, mild to moderate hypercarbia may be tolerable in most cases; however, for a long-duration application such as in this case, the resulting marked hypercarbia may limit the duration of safe percutaneous oxygen insufflation, although, with an underlying healthy cardiovascular system, profound respiratory acidosis due to hypercarbia can be tolerated for prolonged periods of time [1,2].
In an in vitro experiment [9], it has been reported that it takes approximately 11.2 s (44.64 ml/s) to passively exit 500 ml of oxygen insufflated through a 14-G catheter under normal lung compliance (100 ml/cmH2O). As the inspiration:expiration ratio is 2 s (for insufflation of 500 ml):11.2 s (for exit of 500 ml), the respiratory rate and minute volume would be 4.5/min and 2,275 ml/min, respectively. Given that the minute volume is 5–8 L/min, hypercarbia cannot be avoidable. However, instead of waiting for SaO2 to drop by 93% in Wexler et al.’s case [19] in which it took 6–8 min to drop to 93%, if we adjust inspiration and expiration time in a ratio of 2:10 as described above, hypercarbia may occur in a less severe form. However, it is difficult to maintain this ratio in reality. Adjusting inspiration and expiration time by watching the chest wall can be another option, but this method is not suitable because the expired volume that passively comes out through the 14-G cannula is too small. Conversely, it may be preferable to use 12-G (ID: 2.2 mm) or 10-G (ID: 2.6 mm) as a transtracheal cannula, although it has the disadvantage of a large outer diameter (12-G: 2.8 mm, 10-G: 3.4 mm). When 12-G was used as a transtracheal cannula, the CO2 level was maintained at twice the control level (~60 mmHg) [7], which seems to be acceptable. In particular, if 10 G is used, the CO2 level is expected to be even lower. While it is very difficult to squeeze the Ambu bag through the 12-G cannula, it may not pose any problems to insufflate the oxygen volume of 500 ml at an oxygen flow rate of 15 L/min with Rapid-O2 through the cannula. Due to the difficulty of bagging with the 12-G cannula, a breathing rate of 10–12/min was maintained in Coté et al.’s study [7], which means that it takes approximately 6 s for one breath. Therefore, insufflation of 500 ml of oxygen for 2 s followed by expiration for 4 s would be acceptable for ventilation.
JET VENTILATION USING DUAL LUMEN CATHETER (INFLOW LUMEN, OUTFLOW LUMEN)
Complete upper airway obstruction is a contraindication for percutaneous transtracheal jet ventilation. In this situation, the injection of high-pressure gas into the trachea can result in barotrauma because expiration is entirely passive and dependent on the compliance of the lung parenchyma and chest wall. Accordingly, transtracheal jet ventilation should be avoided in cases of complete upper airway obstruction. Dworkin et al. [20] reported that if the effective tracheal diameter is larger than 4.0 to 4.5 mm, regardless of all other lung/jet ventilating factors, air trapping would not occur. The greater the expiratory obstruction, the lower is the need for jet-type ventilation.
Rone et al. [21] demonstrated that normal blood oxygenation and ventilation can be maintained using a dual-lumen catheter with 16-G inflow lumen and 10-G outflow lumen when complete airway obstruction is artificially created in an anesthetized and paralyzed 27-kg dog. The application of this dual lumen may enable the use of jet ventilators in complete airway obstruction, however, the addition of outer diameters of 16-G (outer diameter: 1.7 mm) and 10-G (outer diameter: 3.4 mm) results in a large outer diameter of 5.1 mm. With such a large outer diameter, the puncture of the cricothyroid membrane is a burden. To the best of our knowledge, there have been no reports on the application of the dual lumen in humans.
CONCLUSION
In near-complete or complete airway obstruction, applying Rapid-O2 is a simple and effective maneuver to achieve re-oxygenation for a short period of time. However, prolonged use may result in marked hypercarbia. In contrast, Ventrain® can be used effectively for both oxygenation and ventilation, although the use of this device is slightly more complicated than that of Rapid-O2. Among the devices developed to date for use in complete airway obstruction, Ventrain® appears to be the most ideal.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
Data generated or analyzed in this study are included in this published articles listed in references.
AUTHOR CONTRIBUTIONS
Writing - original draft: Hye Jin Kim. Writing - review & editing: Hyun Joo Kim, Wyun Kon Park. Supervision: Hyun Joo Kim, Wyun Kon Park.
