Aprepitant prophylaxis effectively reduces preventing postoperative nausea and vomiting in patients receiving opioid based intravenous patient-controlled analgesia
Article information
Abstract
Background:
Aprepitant is effective in prevention of chemotherapy-induced nausea and vomiting, when administrated with other antiemetics. We compared the effectiveness of aprepitant to ondansetron for prevention of post-operative nausea and vomiting (PONV) in patients who received a patient-controlled analgesia (PCA) containing opioids.
Methods:
198 patients were randomized into two groups. The treatment group was received an aprepitant, 80 mg, and the control group received a placebo. General anesthesia with inhalational anesthetics–N2O was performed, and PCA was supplied, which contained opioids-NSAIDs-ondansetron. The primary end-point was the incidence of PONV for postoperative 48 hours, and the secondary end-point was the changes in the relationship between PONV incidence and risk factors.
Results:
PONV incidence in the treatment group was lower than in the control group (18.6% [95% CI: 10.8–26.3], 33.3% [95% CI: 23.6–43.1], respectively, P = 0.021). Relative risk of PONV in the control group was 1.80 (95% CI: 1.08–3.00, P = 0.010). PONV scores peaked at around postoperative 6 hours, then gradually decreased in the control group but not in the treatment group, which showed lower values than the control group (P = 0.001), and no changing patterns were observed (P < 0.001). Risk factors analyzed were sex, surgery type, history of motion sickness or PONV, and smoking habits. Their effects of all risk factors except sex were abolished in the treatment group.
Conclusions
Prophylactic aprepitant with ondansetron was more effective than ondansetron-only regimen in preventing PONV after volatile anesthesia with opioid-containing PCA. Aprepitant abolished the effects of most of risk factors, so it could be efficacious in a high-risk PONV group.
INTRODUCTION
Opioid-based, intravenous, patient-controlled analgesia (PCA) is a popular and effective method for postoperative pain control. However, postoperative nausea and vomiting (PONV) is one of the intractable complications of general anesthesia, and its incidence associated with opioid use reached up to 79% [1–4]. Various antiemetics in conjunction with PCA have been introduced to reduce PONV; however, the result of PONV prevention has not been satisfactory [5,6]. Among these antiemetics, because of its prophylactic efficacy and its cost effectiveness, ondansetron, 5-hydroxytryptamine receptor 3 (5-HT3) antagonist has already been established in various surgical procedures requiring general anesthesia. However, many clinical trials reported that ondansetron was not enough for reducing PCA related PONV [7,8].
Aprepitant is a neurokinin-1 (NK1) receptor antagonist that blocks both the central and peripheral emetic stimuli of substance P [9] with a long half-life (9 to 12 hours). With combination therapy, aprepitant is approved for use and recommended for the prevention of PONV [10–13]. It is highly expected that it could cover the short action duration of ondansetron. The purpose of this study was to determine whether or not a single-dose aprepitant prophylaxis before an operation could be beneficial in reducing PONV in patients using an opioid-based intravenous PCA that contains ondansetron alone.
MATERIALS AND METHODS
Before the commencement of the study, appropriate hospital Institutional Review Board approval (GR08120-003) and written informed consent were obtained. We included 267 patients for eligibility. They were between the ages of 19 and 65, met criteria of the American Society of Anesthesiologists physical status classification of I or II, and were scheduled to receive elective surgery under general anesthesia, including nitrous oxide with volatile anesthesia. They all selected PCA as a postoperative pain control. We excluded patients who were pregnant, breast-feeding, or undergoing surgery with regional anesthesia or total intravenous anesthesia. Patients who needed to keep an endotracheal tube or naso-gastric tube were also excluded, as were those undergoing surgeries including eyeball, airway, middle ear, brain, and thorax. We excluded those who experienced vomiting of any organic etiology during the preoperative period or had abnormal laboratory values (alanine aminotransferase [AST] or aspartate aminotransferase [ALT] > 100 IU/L, bilirubin > 1.8 mg/dL, creatinine > 1.6 mg/dL) and QT prolongation, atrial fibrillation, or coronary problems on electrocardiogram. Patients who received medications metabolized by cytochrom P450 3A4 (CYP3A4) were excluded. Eventually, we enrolled 198 patients to two groups randomly, using a computer-generated random number table. The patients in the treatment group (n = 103) took single-dose oral aprepitant 80mg with a small amount of clear water 1 hour before induction of anesthesia. Meanwhile, the patients in the control group (n = 95) were administrated a placebo 1 hour before induction of anesthesia. All procedures were carried out by 2 independent anesthesiologists not involved in the study. A blinded independent anesthesiologist prepared the aprepitant 80 mg and the placebo, which was the same shape as the aprepitant 80 mg capsule with a sealed opaque envelope, and another blinded independent anesthesiologist administrated to the patients. These two anesthesiologists did not participate in any remaining study process. Before the operation, we recorded patients’ histories, such as motion sickness, PONV, and smoking, which could affect PONV. The scheduled surgery was also recorded and subdivided into surgery, including of the abdominal area and surgery involving peripheral extremities and the abdominal area. Surgeries for fractures involving upper and lower extremities, arthroscopic surgery, arthroplasty, tendon release, and others were categorized intro surgery involving peripheral extremities and not the abdominal area. Premedication with intramuscular injection of glycopyrrolate 0.2 mg and midazolam 2 mg was done to both groups. General anesthesia was induced with thiopental (4–5 mg/kg) or propofol (1.5–2 mg/kg) and neuromuscular blocking agents. Anesthesia was maintained with nitrous oxide (40%–60%) with volatile anesthetics. And a neuromuscular block was fully reversed with intravenous pyridostigmine and glycopyrrolate prior to extubation of the trachea (train of four > 0.9). At the end of surgery, we connected PCA to the patients of two groups. The PCA regimen consisted of 700 μg fentanyl, 4 mg hydromorphone, 150 mg ketorolac, and 16 mg ondansetron. A PCA infuser (AP 0605 Ana plus®, EWHA meditech, Korea) was made to deliver about 0.5 ml/hour as continuous infusion and 0.5 ml per demand with a 15-minute lockout for 48 hours period. A bolus injection to increase the opioid plasma concentration level up to adequate analgesia was prepared with the dose of 50 μg fentanyl, 0.3 mg of hydromorphone, and 10 mg ketorolac, and this was administrated within postoperative 1 hour.
Investigators who were blinded to the patient’s group assessed the incidence and severity of nausea, vomiting, and pain intensity at 0.5, 1, 2, 6, 12, 24, and 48 hours postoperatively. The severity of nausea and the use of additional antiemeitics were recorded. Nausea severity scores were expressed using an 11-point verbal rating scale, with 0 meaning no nausea and 10 meaning worst possible nausea. Vomiting or retching episodes were also recorded separately. Pain intensity scores were measured on a visual analogue scale (VAS), with 0 cm meaning no pain and 10 cm meaning worst pain imaginable. Additional antiemetic therapy was given to patients who showed more than one episode of emesis at a time or nausea lasting longer than 15 minutes, and at their request.
The patients could receive another pain control medication if they complained of pain ≥ 5 cm on the VAS. During the study period, the occurrence of frequently reported side effects of aprepitant, such as asthenia, fatigue, hiccups, constipation, diarrhea, and anorexia was assessed. Laboratory adverse events, such as proteinuria, increase of ALT, AST, blood urea nitrogen, and serum creatinine, were assessed using postoperative laboratory data.
For blindness during the study period, all case reports were kept in closed envelopes until statistical analysis.
The primary end-point was the overall incidence of the two groups during postoperative 48 hours. The secondary end-point was the relationship changes between PONV incidence and the risk factors according to aprepitant prophylaxis.
All data was expressed as mean ± standard deviation or an estimated value with a 95% confidence interval (95% CI). All variables were evaluated for normality using the Shapiro-Wilk test. Statistical analysis was performed using chi-square test for categorical variables, z-test for rate, and t-test or two-way repeated measures analysis of variance (RM-ANOVA) for numerical variables. For two-way RM-ANOVA, the sphericity assumption was evaluated with Mauchly’s test, and according to those results, the Greenhouse-Geisser correction was performed. Correlation analysis was also performed to evaluate risk factors. Under 0.05 of α error probability was regarded as statistical significance. All statistical analysis was performed using IBM SPSS 23 for windows (IBM Co., USA) and Microsoft Office Excel 2010 (Microsoft Corp., USA).
The study was powered to achieve at least 80%. The assumption ran as follows: 2-sided significant level of 0.05, the probability of PONV incidence of 40% in the control group and of 30% in the treatment group. Under the assumption of the 2 distribution, the calculated effect size was 0.204 and the required sample size was 189. We presumed that the expected exclusion rate was 30% during patient enrollment and 10% during the rest of the period of the study. As a result, the total required sample size for eligibility assessment reached 250.
RESULTS
Among the 267 patients who were assessed for eligibility, 69 patients were excluded due to exclusion criteria and patient refusal. In all, 198 patients were allocated into two groups according to computer generated randomization numbers. Two patients received neither the aprepitant nor the placebo, due to vomiting induced by unscheduled nasogastric tube insertion. One patient in the treatment group was also excluded because the scheduled operation was delayed for a few hours. During postoperative periods, uncovered blindness occurred in 3 patients, and one patient refused a follow-up visit for assessment of PONV. Penultimately, 189 patients were included in the statistical analysis. After uncovering blindness, an additional 2 patients were excluded due to vague records and missing data in case report forms. Finally, 187 patients were included in the statistical analysis (Fig. 1).
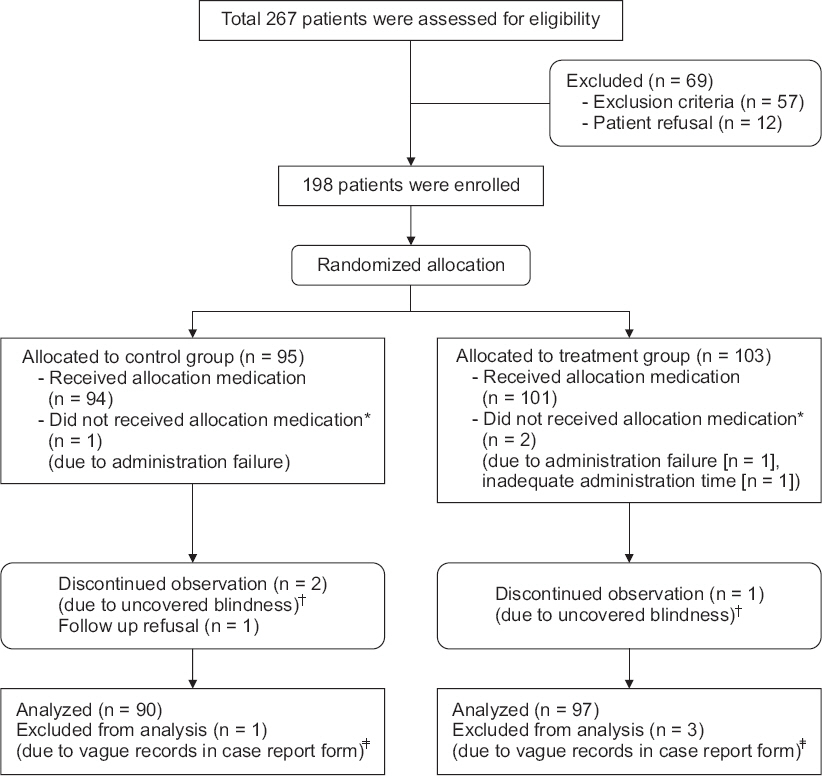
Study flowchart. *Two patients were not received aprepitant or placebo due to vomiting induced by unscheduled nasogastric tube insertion. One patient in treatment group was also excluded because the scheduled operation was delayed for few hours. †They are received active aprepitant or placebo adequately, but blindness was not kept to the physician who monitored study patients during check procedure of obscure documentation about randomization number in case report form. ‡Incorrect recording method or inaccurate writing on case report form.
There were no statistical differences between control and treatment groups in patient characteristics (Table 1).
The proportions of PONV incidence varied in the two groups. Thirty patients presented PONV in the control group (n = 90) and 18 patients in treatment group (n = 97). The proportions of presenting PONV were significantly related to the groups (P = 0.032). Overall, PONV incidences during the 48 hours after surgery were significantly different between the two groups (P = 0.021). The incidence was 33.3% in the control group (95% CI: 23.6–43.1) and 18.6% in the treatment group (95% CI: 10.8–26.3). The relative risk of PONV in the control group compared to the treatment group was 1.80 and 95% CI ranging from 1.08 to 3.00 (P = 0.010). The achieved power was 0.989 under the expected incidences as above.
The PONV score in the control group peaked at around 6 hours from the end time of the surgery, after which it decreased as time went by. But in the treatment group, the PONV scores during the observation periods remained at lower levels, without significant changes, even at around 6 hours from the end time of the surgery (P = 0.002). The overall PONV score in the treatment group was significantly lower than that in the control group and would have been so even if the time effect were abolished (P = 0.002, Table 2).

Overall Incidence and Verbal Rating Score of Post-operative Nausea and Vomiting (PONV) Changes during Postoperative 48 Hours
During the 48 hours after surgery, there were no differences in pain scores between two groups (P = 0.260). The mean pain scores gradually decreased over time (P < 0.001, Table 3).
The incidences of vomiting or retching were also significantly higher in the control group than in the treatment group (P = 0.001). The incidences of vomiting or retching during the postoperative 48 hours were 27.8% (95% CI: 18.5 – 37.0) in the control group and 9.3% (95% CI: 3.5–15.1) in the treatment group. The relative risk of vomiting or retching in the control group compared to the treatment group was 3.0 (95% CI: 1.5–6.1, P < 0.001).
Correlation analysis revealed significant risk factors of PONV (Table 4). Following these results, we decided the risk factors of PONV were sex, surgery type, history of motion sickness or PONV, and smoking habits. Compared to the correlation coefficients of these four risk factors, aprepitant prophylaxis more strongly correlated with a reduced incidence of PONV. In the subgroup analysis, all 4 risk factors presented significant correlation with PONV in the control group. However, only sex significantly correlated with PONV in the treatment group (Table 4).
There were no serious aprepitant-related complications during the study periods, including asthenia, fatigue, hiccups, constipation, diarrhea, and anorexia, and there were no laboratory adverse events, such as proteinuria, increase of ALT, AST, blood urea nitrogen, and serum creatinine, which were apparently related to the use of aprepitant.
DISCUSSION
In this randomized, double-blind, active-control case study, in our efforts to identify to what extent single-dose aprepitant prophylaxis before general anesthesia reduced PONV in patients using an opioid-based PCA containing ondansetron, we could observe that aprepitant prophylaxis effectively reduced the incidence and severity of PONV, and aprepitant prophylaxis has a potential for modify the effect of the risk factors that were correlated with the incidence of PONV.
The pathogenesis of PONV is complicated because of the involvement of multiple receptors and stimuli. Neurotransmitter receptor systems, such as dopaminergic (D2), cholinergic (muscarinic), histaminergic (H1), serotonergic (5-HT3) and NK1 systems mediate the signals leading to PONV [10,14,15]. Besides, PONV depends on various factors, including types of anesthesia or surgery, patient characteristics, and the contents of a PCA. The presentation of PONV may take place early, occurring up to 6 hours after the end of surgery, or late, occurring up to 24 or 48 hours after the end of surgery. The use of volatile anesthetics may be a main cause of early PONV, and opioid-induced symptoms may account for much of late PONV [6,12,16]. In this study, PONV incidence still reached up to 33% in a group supplied with only ondansetron (5-HT3 receptor antagonist). The severity of nausea in this group increased until 6 hours postoperatively, after which it decreased gradually. This result is consistent with those of earlier studies, which reported that ondansetron alone was not enough for prevention of PONV [7,17].
Aprepitant is approved for use and recommended in consensus guidelines for the prevention of chemotherapy-induced nausea and vomiting [18]. Considering more than half of the patients who receive chemotherapy experience chemotherapy-induced nausea and vomiting, we hypothesized that a single dose of oral aprepitant prophylaxis before an operation, in conjunction with ondansetron in PCA, could be an effective regimen to alleviate PONV. Aprepitant is a highly selective, brain-penetrant NK1 receptor antagonist with a long half-life and preclinical efficacy against opioid-induced emesis [19–21]. Aprepitant can reduce the emetic effect of substance P, which is found in the gastrointestinal tract and areas of the central nervous system thought to be involved in the vomiting reflex. In our study, the patients who were administered aprepitant prophylaxis before anesthesia, in conjunction with ondansetron, showed a greatly reduced PONV incidence, at 18.6%, and a score of PONV maintained at a lower level without significant changes during the postoperative 48 hours. This is in close agreement with the results of previous studies, which reported that aprepitant was superior to ondansetron alone for the prevention of vomiting, especially in the first 24 and 48 hours postoperatively [9,11,12].
The advantages of aprepitant for PONV include its oral formulation and its easy administration for prophylaxis, along with the premedication. Also, due to the long-lasting effects of this drug, up to 24 hours, it can have advantages against PONV and efficacy against opioid-induced emesis.
The reported incidence PONV associated with PCA opioids varies between 30% and 70% [1,2]. These undesirable side effects increase greatly in groups at high risk for PONV. They include female sex, surgery type, a history of motion sickness, previous PONV, nonsmoker, etc. [22,23]. It is thought that aprepitant could be beneficial in these groups of patients when they are using PCA. According to correlation analysis, type of surgery (surgery involving abdominal area), history of motion sickness, or previous PONV, female sex, current smoking habit, are the identified risk factors for PONV. However, in patients with these risk factors, aprepitant prophylaxis was most strongly and significantly correlated with PONV incidence reduction. This means that aprepitant prophylaxis could have a potential role in ameliorating the effects of other risk factors related to the occurrence of PONV. Subgroup analysis results revealed that correlation coefficients of type of surgery, history of motion sickness or PONV, and current smoking habit showed insignificant statistical results. Only sex was statistically significant.
Previous trials about the effect of aprepitant in preventing PONV revealed that 40 mg aprepitant was sufficient to prevent PONV effectively [11,12]. All patients enrolled in our study were more exposed to risk factors for PONV, including potent halogenated inhalational anesthetics with nitrous oxide and continuous infusion of opioids from intravenous PCA, than those in other clinical trials. The situation is not exactly same with regard to exposure to emetogenic chemotherapy. In that case, we assumed that an opioid-containing intravenous PCA delivers similar results in mild to moderate emetogenic situations, and an 80 mg aprepitant instead of 40 mg could be better in achieving desirable results. Moreover, in our country, only 80 mg and 125 mg capsular forms of aprepitant were commercially available, so we decided to use the 80 mg single-dose prophylactic aprepitant with ondansetron for PONV prophylaxis with expectation of a nearly complete response.
For using an active control-case design and avoiding ethical issue, ondansetron was administered to both groups, and this was a limitation of this study. However, considering the duration or the effects of ondansetron, aprepitant prophylaxis was expected to reduce PONV by a considerable degree, compared to other risk factor modifications. Also, this study is expected to result in a more generic consequence, as it covers most eligible types of surgery that are subject to a study period and is not confined to a specific surgery type. Due to exclusion criteria, we excluded the majority of upper abdominal surgeries, which require a naso-gastric tube. Surgery involving the abdominal area mostly included lower abdominal surgery, and gynecological surgery. The peripheral surgery types were orthopedic and plastic surgery involving extremities, including arthroplasty, fracture fixation, and so on. This would not produce a homogenous result. However, we included high- and low-risk PONV surgical types, and we confirmed that the use of aprepitant prophylaxis could modify the adverse effects of most PONV risk factor, including high-risk surgery.
In conclusion, a single dose of prophylactic aprepitant before an operation could be beneficial in reducing PONV in patients using opioid-based IV PCA containing ondansetron alone. The prophylactic aprepitant with ondansetron seems to be a promising regimen as a prophylactic antiemetic, even in patients with high risk factors for PONV. Its long-lasting effects, up to 48 hours, could be effective not only for early PONV but for late PONV, which is highly correlated with the use of opioids for postoperative pain control.



