Transcutaneous neurostimulatory treatment for peripheral polyneuropathy induced by hypereosinophilic syndrome - A case report -
Article information
Abstract
Background
Hypereosinophilic syndrome is a rare disease that increases the number of circulating eosinophils in the body. It has many complications, including peripheral polyneuropathy. Peripheral polyneuropathy often does not respond well to conventional therapies. Transcutaneous neurostimulatory treatment, also known as scrambler therapy, is an alternative modality for the treatment of chronic retractable pain.
Case
A 47-year-old female presented with complaints of bilateral calf pain. She had been under treatment for peripheral polyneuropathy induced by hypereosinophilic syndrome for 7 years. Pharmacologic treatment did not affect the patient’s symptoms.
Conclusions
Transcutaneous neurostimulatory treatment was administered to the patient. It was effective on her symptoms, and the effect of pain alleviation continued for 3 months.
Hypereosinophilic syndrome (HES) is characterized by an increase in the number of circulating eosinophils [1]. Patients with HES may present with various combinations of symptoms and signs of organ damage mediated by eosinophils, including dermatological, pulmonary, gastrointestinal, cardiac, and neurologic dysfunctions [2]. Four percent of all HES patients experience neurological manifestations, and peripheral polyneuropathy (PN) accounts for approximately half of these neurologic manifestations. Peripheral neuropathy can be classified into various types. For example, they can be symmetric or asymmetric, involve sensory nerves with or without involvement of motor nerves, and can cause mononeuritis multiplex or radiculopathy [3]. However, the pathophysiology of PN in patients with HES remains unclear. Therefore, there is no specific treatment for PN in patients with HES. Transcutaneous neurostimulatory treatment (TNT), of which scrambler therapy® (ST) is the most well-known, has been used to treat chronic pain syndrome since 2003, when Marineo [4] suggested that ST was effective in patients with terminal cancer pain. The mechanism of TNT involves the production of 16 different electrical currents that stimulate normal nerve action potentials and replace “pain” with “non-pain” signals via noninvasive electrodes placed around the surface of painful areas [5]. This treatment has been utilized for the treatment of chronic intractable pain syndromes such as chemotherapy-induced neuropathy, postsurgical pain syndrome, or postherpetic neuralgia [6-8]. We recently experienced a case of successful treatment with Pain Block® (PB) (Koibig Inc., Korea) for TNT in a HES patient with peripheral polyneuropathy. Prior to this case, there were no reports of TNT in patients with HES.
CASE REPORT
Written informed consent was obtained from the patient for the publication of this case.
A 47-year-old female patient (height, 164 cm; and weight, 60 kg) visited our pain clinic for pain in both calves. She had been diagnosed with HES four years ago. She was referred from the hemato-oncology clinic because her pain was uncontrolled with pharmacological treatment, despite continuous treatment for a few years. She had no medical history except for HES and denied smoking or alcohol history. The degree of pain on the 1–10 numeric rating scale (NRS) was eight. She complained that she experienced several daily episodes of breakthrough pain increasing to NRS values of 9–10, and described the pain as a continuously tingling and stabbing pain. She did not have any motor weakness or paresthesia. Her Neuropathic Pain Scale (NPS) score was 61.0, and she had uncontrolled, severe neuropathic pain.
The location of the pain did not follow specific dermatomes, and it was expressed from both knees to the tip of the feet, entirely. Magnetic resonance imaging was performed to differentiate it from other diseases. However, she had only mild Achilles paratenonitis on both sides with no other specific lesions. Both calf muscle biopsies showed focal mixed inflammatory cell infiltration into the endomysium, forming inflamed granulation tissue with many eosinophils. At the first visit to our pain clinic, the patient had been administered oxycodone 5 mg bid, pregabalin 300 mg tid, acetaminophen 325 mg bid, tramadol 37.5 mg bid, and amitriptyline 10 mg qd. Tapentadol IR 50 mg was administered as rescue medication. However, she complained that the medication had an insufficient effect on her severe pain, and that she could not sleep very well at night. Therefore, we concluded that the patient needed additional treatment, and TNT was planned.
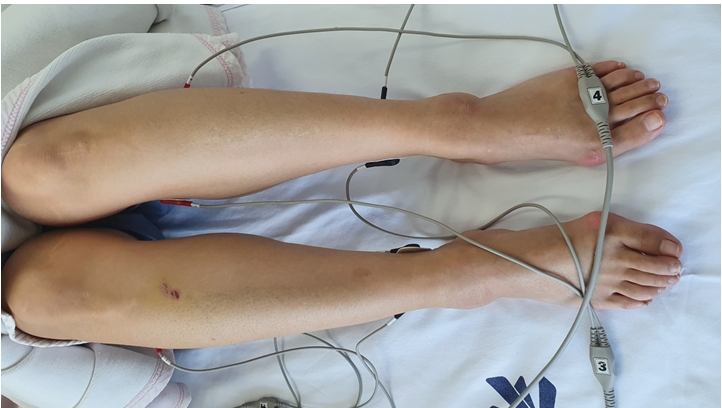
According to our hospital Institutional Review Board (IRB) regulation, Case report is not reviewed by IRB committee. As the patient’s pain was located below the knees, the PB electrodes were attached from both knees to the soles. (Figs. 1, 2) The detailed protocol is as follows: First, we clearly defined the area of pain on the patient before the start of TNT. Next, electrodes were attached to the areas proximal and distal to the margin of the area of pain. The PB was then turned on, and the electrode intensity was increased to the maximum tolerated intensity. Each session lasted for 40 min, and the entire treatment lasted for 15 consecutive sessions. However, a two-day interruption was considered acceptable. The dial of the PB can be adjusted from 0% to 100%. At the highest setting of 100% on the dial of PB, the amperage is 4.9–9.1 mA and the voltage is 7.4–13.8 V. In the case of this patient, a dial value of 35–50% was applied.
During PB treatment, the NRS score decreased to 0 or 1. The NRS score was maintained at 3 between visits to the pain clinic. The patient received a total of 15 sessions of PB treatment for five weeks. The area of pain did not decrease, but she reported disappearance of the breakthrough pain as well as NRS scores of 0 or 1 during treatment and 3 or 4 at home. Pharmacologic treatment was continued without a change in dosage but the patient did not take further rescue medication. The pain alleviation effect from the PB treatment continued for 2 months, during which breakthrough pain did not develop (Table 1). Her NPS score lowered to 16.0; however, during the third month after treatment, the pain recurred in the same area. She complained of an NRS score of 6, which was lower than that before the initial treatment. She has been receiving the previous PB treatment again, and her NRS score has decreased to 2.
DISCUSSION
HES is a group of disorders marked by the sustained overproduction of eosinophils, in which eosinophilic infiltration and mediator release cause damage to multiple organs [9]. Blood eosinophilia is defined as an absolute eosinophil count of > 1.5 × 109/L, and HES is defined as a consistent blood eosinophilia and subsequent end organ dysfunction without evidence of an underlying cause. HES is initially treated with glucocorticoids. If the patient is steroid-unresponsive or has a special type of HES with Fip1-like 1-platelet-derived growth factor receptor alpha, imatinib mesylate may be administered [9,10].
In this case, TNT with PB is an effective treatment for PN induced by HES that does not respond to pharmacological treatment. HES is a very rare disease and its prevalence is not well known. In a study analyzing the database of the Surveillance, Epidemiology, and End Result program for cancer, the estimated prevalence was between 0.36–6.3 per 100,000 [11]. The mechanism by which PN appears in HES is still unclear, but it is thought that eosinophil deposition plays a major role in other complications and causes axonal degeneration with neurogenic atrophy of muscle [12]. As far as we know, there have been no reports on the specific treatment for PN induced by HES. Therefore, we suggest that the application of TNT could be expected to be effective, as in this case, where there are repetitive complaints or retractable PN despite pharmacological treatment.
Conventional treatments for peripheral polyneuropathy include pharmacologic treatment, such as anticonvulsants and antidepressants, and nerve blocks including central or peripheral nerve block. However, in this patient, pharmacologic treatment was not effective, and we did not consider nerve block because the patient’s symptoms did not follow a specific dermatome nor were they limited to the innervation area of a specific peripheral nerve. Although there have been no randomized controlled trials for the treatment of peripheral polyneuropathy, several studies have reported effective treatments for PN induced by chemotherapy or diabetes mellitus. As in our case, those studies showed that application of TNT to patients with PN was effective in relieving their pain, and they showed successful results during the application of TNT and in the follow-up period [13–15].
The mechanism underlying TNT is not clearly understood. According to Marineo [4], TNT provides “non-pain” information to the peripheral sensory nerve receptors, which is conveyed to the central nervous system through C-fibers and Aδ-fibers. Subsequently, it reduces pain [4,14]. Fig. 1 demonstrates the basic application of TNT in a patient. Before the first treatment with TNT, the pain area was clearly defined, and electrodes were attached to the areas proximal and distal to the margins of the pain area. Next, an electrical stimulus was applied, and the intensity was slowly increased until the patient could not tolerate the pain stimulation. Each treatment took approximately 40 min, and all consecutive sessions needed to be done approximately 10 to 15 times.
TNT differs from transcutaneous electrical nerve stimulation (TENS), which is usually used in physical therapy. TENS provides an on-off biphasic current without variation, whereas TNT provides continuously changing variable nonlinear waveforms. In addition, the pulse rate of TNT is 43–52 Hz, and the mean energy delivered per second is generally less than that of standard TENS devices [6].
In TNT, ST is the representative choice ever since Marineo [4] first reported its application in a clinical situation. The PB used in this case is similar to ST at a pulse rate of 43–52 Hz. However, PB differs from ST in its pulse waveform. ST generates 16 specific types of waveforms, while PB generates a random waveform using a random variable program. This PB is a TNT product approved by the Korean Ministry of Food and Drug Safety for refractory pain, chronic pain, and cancer pain. Nevertheless, further prospective studies are required because the possible differences between the existing ST and PB in actual clinical situations are not yet known.
We found TNT to be a clinically useful, non-invasive therapeutic modality. It should be considered as an effective alternative measure for treating PN induced by HES. Further studies including randomized controlled trials are needed to confirm and generalize our findings.
Notes
CONFLICTS OF INTEREST
In this case, the medical machine was provided as the hospital-based business program.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
AUTHOR CONTRIBUTIONS
Writing - original draft: Ji Eun Park, Woosoo Park, Teakseon Lee. Writing - review & editing: Kihyug Kwon. Investigation: Woosoo Park, Teakseon Lee. Supervision: Kihyug Kwon.