Effects of 10-min of pre-warming on inadvertent perioperative hypothermia in intraoperative warming patients: a randomized controlled trial
Article information
Abstract
Background
This study aimed to evaluate the efficacy of 10-min pre-warming in preventing inadvertent perioperative hypothermia, which is defined as a reduction in body temperature to less than 36.0℃ during the perioperative period in intraoperative warming patients.
Methods
In this prospective randomized study, 60 patients scheduled for elective surgery under general anesthesia lasting less than 120 min were divided into two groups: the 10-min pre-warming group (n = 30) and the control group (n = 30). Patients in the 10-min pre-warming group were pre-warmed for 10 min in the pre-anesthetic area using a forced-air warmer set at 47ºC. Intraoperatively, we warmed all patients with a forced-air warmer. Body temperature was measured using a tympanic membrane thermometer pre- or postoperatively and a nasopharyngeal temperature probe intraoperatively. Patients were evaluated on the shivering and thermal comfort scale in the pre-anesthetic area and post-anesthesia care unit.
Results
The incidences of intraoperative hypothermia and postoperative hypothermia were similar in both groups (10.7% vs. 28.6%, P = 0.177; 10.7% vs. 10.7%, P = 1.000 respectively). Body temperature was higher in the 10-min pre-warming group (P = 0.003). Thermal comfort during the pre-warming period was higher in the 10-min pre-warming group (P < 0.001). However, postoperative thermal comfort and shivering grades of both groups were similar.
Conclusions
Ten minutes of pre-warming has no additional effect on the prevention of inadvertent perioperative hypothermia in intraoperative warming patients.
INTRODUCTION
Perioperative hypothermia, defined as a core body temperature below 36ºC, commonly occurs at a rate of 50–90% even in simple surgical procedures. It is associated with multiple perioperative complications and should be prevented [1–3]. Recently, various methods of active intraoperative warming for minimizing perioperative hypothermia have been implemented, forced-air warming is the most commonly used, as it is simple, safe, and effective [4]. However, hypothermia occurs in approximately 65% of patients within the first hour of surgery even with active intraoperative warming, and additional preventive measures are required [5].
Perioperative hypothermia that occurs within the first hour of surgery develops immediately after the induction of anesthesia, leading to a 1.6ºC decrease in the core temperature. Of this decrease, 81% is attributed to the core-to-peripheral redistribution of body heat due to anesthetic-induced vasodilation; 46 kcal of heat is redistributed [6]. This redistribution hypothermia can be prevented by increasing the peripheral temperature and decreasing the core-periphery temperature gradient through pre-warming [4,7]; several guidelines recommend at least 30 min of pre-warming [8,9]. However, it is difficult to apply the recommended ≥ 30 min of pre-warming when there is inadequate or no preoperative holding area.
Recently, there have been reports that pre-warming with a duration shorter than 30 min is effective. Brauer et al. [10] reported that ≤ 30 min of regular pre-warming effectively reduced perioperative hypothermia. Horn et al. [11] compared groups that received 10, 20, and 30 min of pre-warming with the control group and reported that 10 min of pre-warming effectively reduced perioperative hypothermia as well. This is corroborated by previous study conducted by our team that compared 10 and 30 min of pre-warming and showed that 10 min of pre-warming is effective [12]. However, most studies on short-term pre-warming applied intraoperative warming only in hypothermic patients, and the effects on patients undergoing continuous intraoperative warming cannot be verified. Furthermore, the effects of pre-warming on patients undergoing continuous intraoperative warming are still controversial, given findings from the existing ≥ 30 min pre-warming cases [13–16].
Thus, the present study aims to investigate whether the 10-min pre-warming, which was recently reported to be effective, provides an additional benefit to patients undergoing continuous intraoperative warming.
MATERIALS AND METHODS
This prospective randomized study was approved by the Institutional Review Board of this institution (no. SCHUH 2017-11-013-001) and registered with the Clinical Research Information Service in Korea (cris.nih.go.kr, no. KCT 0002803). The study period spanned April 19, 2018 to May 11, 2018. Patients visited the facility on the day before the surgery, and informed consent was obtained from every patient.
Subjects
Patients were included in this study if they were 19 years or older, had an American Society of Anesthesiologists physical status (PS) classification of 1–3, and were scheduled to receive ≤ 120 min of elective surgery. Patients were excluded if they had body mass index (BMI) ≥ 35 kg/m2 or preoperative core temperature ≥ 38ºC or ≤ 36ºC, were undergoing regional anesthesia or combined regional and general anesthesia, or were pregnant.
Randomization and masking
Patients were screened on the day before the surgery and randomly assigned to two groups using 1:1 block randomization in Excel (Microsoft Excel 2016; block size 4, 6): 10-min pre-warming group (n = 30) and control group (n = 30). The investigator in charge of the block randomization did not participate in the experiment. An anesthesiologist who had no knowledge of the randomization table conducted the experiment in the operating room and the post-anesthesia care unit (PACU), and a research nurse who knew of the assigned groups conducted the pre-warming and experiment.
General procedures
All patients were trained on the wording of the thermal comfort scale (100-mm visual analog scale [VAS]: 0 mm = the coldest imaginable, 50 mm = comfortable, 100 mm = the hottest imaginable) during their visit on the day before the surgery.
On the day of surgery, patients began receiving fluids stored at room temperature after obtaining intravenous access in the ward approximately 30 min before the surgery. They arrived at the preoperative holding area (same space as the PACU, ambient temperature: 26 ± 1ºC) approximately 10 min before the start of surgery. Immediately after arrival, they were covered with a specialized full-body blanket placed under a cotton blanket, and those in the 10-min pre-warming group received 10 min of pre-warming using a forced-air warmer (Warm TouchTM 6000, Covidien, USA) set at 47ºC, while those in the control group received no forced-air warming. All patients were asked to report to the investigator if they felt discomfort of ≥ 60 on the thermal comfort scale, in which case warming was discontinued or the warming temperature (45ºC, 40ºC) was adjusted depending on the amount of thermal discomfort and the will of the patient. The forced-air warmer used in this study can maintain a warming temperature of 47ºC for up to 45 min, after which the temperature is decreased to 45ºC for patient safety as approved by the Ministry of Food and Drug Safety.
After pre-warming, the forced-air warmer and full-body blanket were removed and patients were transferred to the operating room. The anesthesiologist who had no knowledge of the assigned group performed standard monitoring and induction of general anesthesia using 2 mg/kg of 1% propofol and 0.6 mg/kg of rocuronium, with the patient covered in a cotton blanket. Inhalational anesthetics (sevoflurane or desflurane) and remifentanil were used for maintenance of general anesthesia. Immediately after anesthesia induction, the patient was covered with a specialized blanket for the upper or lower limbs depending on the surgical site, and the surgical site was prepared by the surgeon. After the surgical preparation was completed, continuous intraoperative warming was performed until the end of the surgery. The temperature of intraoperative warming was adjusted, based on the nasopharyngeal temperature, to 45ºC for < 36.5ºC and 40ºC for 36.5–37.5ºC, and warming was discontinued for ≥ 37.5ºC. For all patients, a breathing circuit that allows for intraoperative heating/humidification was used, and no warming devices other than the forced-air warmer for continuous warming were used. After the surgery, pyridostigmine and glycopyrrolate were used to reverse muscle relaxation, and the patient was transferred to the PACU after consciousness and spontaneous breathing were recovered. Immediately on arrival at the PACU, active warming was performed if the patient’s body temperature was ≤ 36ºC, using the forced-air warmer set to 40ºC.
Measurements
Patients were screened on the day before surgery by examining the demographic information, including age, sex, height, weight, BMI, the American Society of Anesthesiologists-PS classification, and the type of surgery.
Upon the patient’s arrival at the preoperative holding area, a research nurse who knew of the assigned group recorded the body temperature measured using a tympanic thermometer (Thermoscan®, infrared tympanic thermometer IRT 4020, Braun, USA) before pre-warming [17]. The tympanic temperature and thermal comfort index were also recorded after pre-warming.
Upon arrival at the operating room, the ambient room temperature was recorded. After securing the airway, a nasopharyngeal probe (ETP1040, Ewha Biomedics, Korea) was inserted 9–10 cm past the nares for intraoperative core temperature monitoring [18]. The time of nasopharyngeal probe insertion was defined as 0 min, and the temperature was recorded every 15 min until the end of surgery. After the surgery, the duration of anesthesia, time from arrival at the operating room to the resumption of continuous intraoperative warming, and operating room temperature at departure were recorded. Upon arrival at the PACU, the tympanic temperature, thermal comfort, and postoperative shivering on a 3-point scale (0 = no shivering; 1 = intermittent, low intensity; 2 = moderate shivering; 3 = continuous intense shivering) were measured and recorded every 10 min.
The primary finding of this study was the incidence of hypothermia, which was defined as a perioperative body temperature below 36.0℃. The secondary findings were the perioperative changes in body temperature, pre-warming-induced thermal comfort, and postoperative shivering and thermal comfort measured in the PACU.
Statistical analysis
Horn et al. [11] reported that the incidence of intraoperative hypothermia requiring intraoperative warming was 31% in the 10-min pre-warming group and 67% in the control group. Therefore, we hypothesized that the 10-min pre-warming in this study would reduce hypothermia at a comparable rate. Given the level of significance of 0.05, the statistical power of 80%, and a dropout rate of 10%, the number of participants for each group was calculated as 30. We used SPSS version 25 (IBM Co., USA) for the statistical analysis. We performed a chi-squared analysis or Fisher’s exact test for categorical variables and the Kolmogorov–Smirnov normality test for continuous variables. We performed the Student’s t-test if the normality assumption was satisfied and the Mann–Whitney test if not. The changes in temperature before, during, and after surgery were analyzed using a mixed-effect model based on a first-order autoregressive covariance structure and graphically represented. If there was a significant temporal difference between the body temperatures of the two groups in the mixed-effect model, we performed a posthoc analysis using the Bonferroni method. Continuous variables were presented as mean ± standard deviation or median with 1Q, 3Q. Categorical variables were presented as frequency and percentage. P < 0.05 was considered statistically significant.
RESULTS
Sixty-seven patients were screened, and three patients who failed to meet the inclusion criteria and four who refused to participate were excluded. Finally, 60 patients were included, and they were randomly assigned to two groups: the pre-warming group (n = 30) and the control group (n = 30). In the 10-min pre-warming group, one patient who refused to receive intraoperative warming due to the anesthesiologist’s mistake and another patient whose intraoperative warming was discontinued at the discretion of the surgeon were withdrawn from the study. In the control group, one patient who requested cancellation right before the surgery and another who showed a high body temperature (> 38ºC) before pre-warming were withdrawn. In the end, data were collected from 56 patients (28 in the pre-warming group and 28 in the control group) and analyzed (Fig. 1).
There were no significant inter-group differences in age, sex, height, weight, BMI, the American Society of Anesthesiologists-PS classification, type of surgery, duration of anesthesia, time from arrival at the operating room to the resumption of continuous warming (duration of unwarming), body temperature on arrival at the preoperative holding area (initial body temperature), and the ambient temperature of the operating room (Table 1).
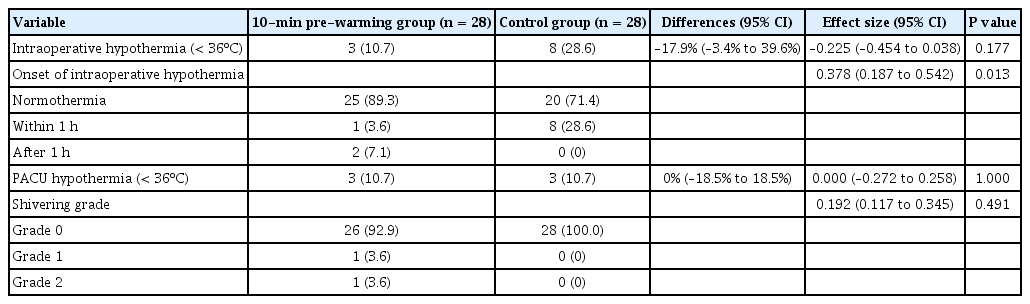
There was also no significant inter-group difference between the incidences of intraoperative hypothermia (10.7% vs. 28.6%; Differences [95% confidence interval, 95% CI] = –17.9% [–3.4% to 39.6%]; φ [95% CI] = –0.225 [–0.454 to 0.038]; P = 0.177). However, the incidence of hypothermia within the first hour was significantly lower in the 10-min pre-warming group than in the control group, and the effect size was large (F = 2, Cramer’s V [95% CI] = 0.378 [0.187 to 0.542]; P = 0.013). There were no significant inter-group differences between the incidences of hypothermia and postoperative shivering measured in the PACU (P = 1.000 and P = 0.491) (Table 2). For all study participants, perioperative hypothermia was mild, measuring between 35.0ºC and 36.0ºC.
There was a significant inter-group difference between the temporal pattern of body temperature changes (P = 0.003), as body temperature was shown to be significantly higher in the pre-warming group from 15 min postoperatively to the end of PACU recovery, excluding the post-induction 120-min time point (P < 0.05) (Table 3, Fig. 2).
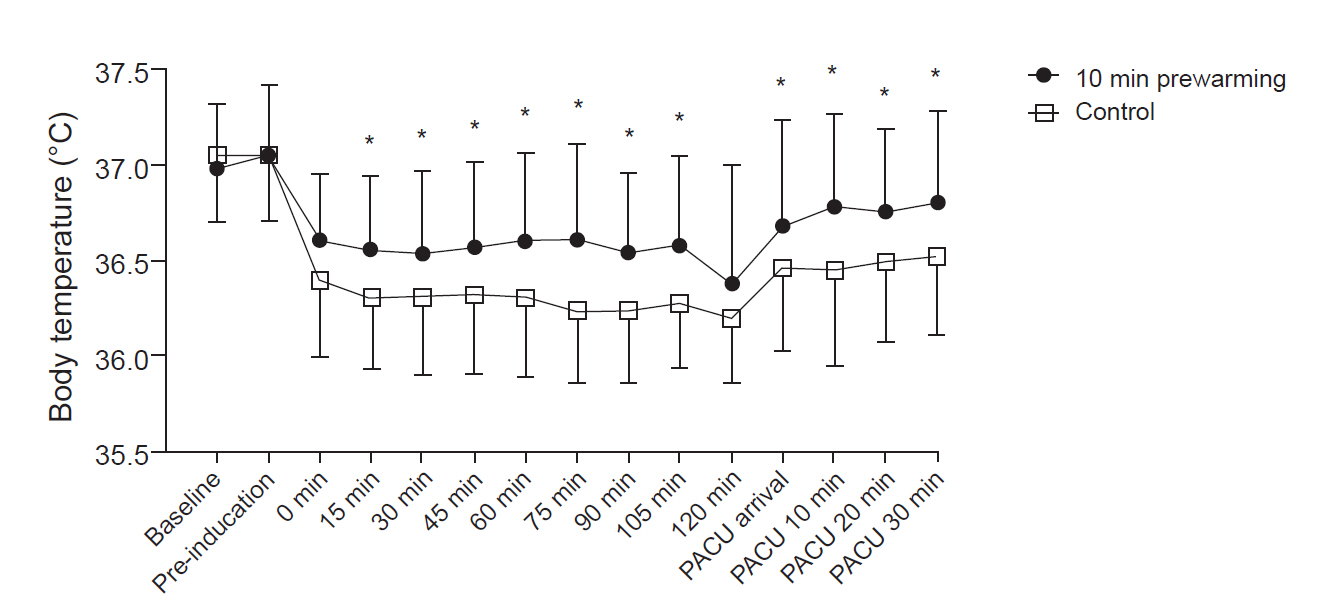
Perioperative body temperature. Preoperative and postoperative core temperatures of the patients were measured using a tympanic membrane thermometer. Intraoperative core temperature was recorded every 15 min after anesthetic induction using a nasopharyngeal probe. The temperature was higher in the 10-min pre-warming group from 15 min after anesthetic induction to the post-anesthesia care unit (PACU) area. Error bars indicate ± 1 SD of temperature at each time. Baseline: immediately after arrival in the preoperative area, Pre-induction: immediately after the end of warming, Intraoperative 0 min: immediately after nasopharyngeal probe insertion, PACU arrival: immediately after arrival at the PACU, PACU 10, 20, 30 min: 10, 20, and 30 min after arrival at the PACU. *P < 0.05 based on post-hoc testing using Bonferroni’s method.
During the duration of pre-warming, the thermal comfort scale was significantly higher in the 10-min pre-warming group (P < 0.001). However, most patients in the 10-min pre-warming group reported the highest score in the thermal comfort scale near the end of pre-warming and did not want pre-warming to discontinue; hence, 10 min of pre-warming at 47℃ was performed in all patients without adjustment. There was no significant inter-group difference between the PACU thermal comfort scores (P > 0.05) (Table 4).
Patients displayed no forced-air warming-induced adverse side effects such as skin symptoms or burns.
DISCUSSION
Many guidelines on the prevention of perioperative hypothermia recommend at least 30 min of pre-warming [8,9]. However, research on the appropriate duration of pre-warming is limited. Sessler et al. [7] reported that a duration of 30 min to 1 h is appropriate, but in this study, pre-warming was performed after 2 h of exposure to a 21ºC environment, which differs from actual clinical scenarios. Pre-warming lasting less than 30 min may be enough in clinical scenarios that do not involve 2 h of exposure at 21ºC [7].
Using higher temperatures for pre-warming may reduce the duration of pre-warming more effectively [7,19]. Sessler et al. [7] also reported that pre-warming at higher temperatures induced greater peripheral vasodilation and heat transfer to the core, although there was no difference in peripheral tissue heat content within the first 40 min. Thus, the temperature of 47ºC used in this study had been used effectively in a previous study without severe thermal discomfort, and it induced greater periphery-to-core heat transfer [12].
Several recent studies have also reported the effect of ≤ 30 min pre-warming. Horn et al. [20] reported that 15 min of pre-warming effectively increased the core temperature in patients undergoing epidural anesthesia (37.1 ± 0.4ºC vs. 36.0 ± 0.5ºC). In a different study, the team reported that in patients undergoing combined epidural and general anesthesia, 15-min pre-warming between the epidural block and general anesthesia effectively reduced perioperative hypothermia (72% vs. 6%) [21]. Shin et al. [22] also reported that in patients undergoing combined brachial plexus block and general anesthesia, approximately 14 min of pre-warming during the block effectively reduced perioperative hypothermia (96.2% vs. 57.7%). However, since these studies involve regional anesthesia, its induced effect of additional peripheral vasodilation cannot be excluded.
Horn et al. [11] reported that 10-min pre-warming effectively reduced perioperative hypothermia in patients undergoing ≤ 120 min general anesthesia. Our team’s previous study also showed that a 10-min pre-warming-induced a reduction in hypothermia comparable to a 30-min pre-warming [12]. However, in these two studies, intraoperative warming was performed only when there was an incidence of hypothermia during surgery; hence, the same effect cannot be expected for patients receiving continuous intraoperative warming.
The effect of pre-warming in patients receiving continuous intraoperative warming has been reported in numerous studies. However, since the incidence of intraoperative hypothermia has decreased with the recent changes in the medical environment, such as the use of laparoscopes and the increase in outpatient anesthesia, the effect is still controversial [13]. Several studies have reported that effective pre-warming reduces the incidence of hypothermia in patients receiving continuous warming [14–16], but Akhtar et al. [13] argued that 60 min of pre-warming at 43ºC did not effectively lower the incidence of hypothermia, which may be explained by the effective passive insulation in the control patients.
The results of this study, consistent with the previous study by Akhtar et al. [13], revealed no significant difference in the incidence of perioperative hypothermia between the 10-min 47ºC pre-warming group and the control group. The double use of the full-body and patient blankets could have induced effective passive insulation in the controls, while the high temperature of the pre-warming area may have also had an effect as well. In compliance with the regulations of the present institution, the ambient temperature of the pre-warming area (same as PACU) was maintained at 26 ± 1ºC. This was higher than the temperature used in previous studies, and Giesbrecht et al. [23] reported that heat loss decreases with increasing ambient temperature.
Unlike the overall incidence of perioperative hypothermia, the incidence of intraoperative hypothermia within the first hour, revealed by posthoc analysis, was significantly lower in the 10-min pre-warming group than in the controls. This suggests that the effect of pre-warming is marked within the first hour of surgery [6].
In this study, the inter-group difference between temperatures during and after surgery, from 15 min post-induction to postoperative recovery, was maintained at ≥ 0.2ºC, which is the clinical temperature difference defined as the warming effect in hypothermic patients by the National Institute for Health and Clinical Excellence (NICE) in the UK [24]; the result was statistically significant. However, there was no statistical difference at 120 min post-induction, which may be because the study was conducted for surgery lasting less than 120 min, and the remaining sample size at 120 min post-induction was small (Table 3). The inter-group differences between temperatures that persisted until recovery may suggest that pre-warming had an effect in addition to the effect of intraoperative warming. This is consistent with previous reports that the temperature difference persisted when intraoperative warming and pre-warming were performed together [14–16,22], while it gradually decreased when intraoperative warming was not performed [11,12].
Sessler et al. [7] reported that when pre-warming was performed at 40ºC and 43ºC, patients complained of thermal discomfort and sweating after an hour. Since we used a high pre-warming temperature of 47ºC, we predicted that patients would complain of thermal discomfort within a short period, and the result showed that thermal comfort was higher in the pre-warming group than in the control group. However, most patients who reported a high thermal comfort index started complaining of high thermal discomfort at approximately 10 min, but they did not wish to discontinue pre-warming to the manageable range, so the experiment was completed as planned.
This study has several limitations. First, the statistical power may have been low due to the small number of study participants. The sample size was calculated based on the study by Horn et al. [11], but the present study employs a design distinct from that of Horn et al. [11]; we performed continuous warming during surgery and maintained a high pre-warming temperature at 47ºC, which may have decreased the overall incidence of hypothermia and resulted in low statistical power. Future research involving a larger sample size is necessary. Second, since this study was conducted for short-duration surgeries lasting ≤ 120 min, the results may not apply to patients undergoing major surgeries lasting more than 120 min. Research on patients undergoing major surgeries should be conducted in the future. Third, the amount of operative blood lost and IV fluid infused, which were not measured in this study, can affect body temperature, which is the main outcome of this study. However, since this study was conducted for surgeries lasting ≤ 120 min, which involve very limited amount of operative blood loss and infusion of IV fluids, the effect is likely minimal. Fourth, the peripheral temperature and heat content were not measured, and they should be estimated. However, since the core temperature is an important indicator for determining perioperative hypothermia and the effect of hypothermia, this should suffice.
In conclusion, 10 min of pre-warming using a temperature of 47ºC did not present an additional advantage for the reduction of perioperative hypothermia in patients receiving continuous intraoperative warming during ≤ 120 min of general anesthesia. However, a higher intraoperative core temperature was maintained with pre-warming relative to the control. Since active and continuous intraoperative warming resulted in a lower incidence of hypothermia in the control group than in previous studies, the effect of pre-warming on the prevention of hypothermia should be verified through further research involving a larger sample.
SUPPLEMENTARY MATERIALS
Supplementary data containing Korean version of this article is available at https://doi.org/10.17085/apm.20027
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: Jae Hwa Yoo, Si Young Ok. Data acquisition: Sun Young Park, Mun Gyu Kim, Ho Bum Cho, Gyu Wan You. Formal analysis: Jae Hwa Yoo, Sang Ho Kim, Ji Won Chung. Supervision: Si Young Ok. Writing-original draft: Jae Hwa Yoo. Writing-review & editing: Jae Hwa Yoo, Si Young Ok, Sang Ho Kim, Ji Won Chung.