The effect of dexmedetomidine and midazolam on combined spinal-epidural anesthesia in patients undergoing total knee arthroplasty
Article information
Abstract
Background
Intravenous dexmedetomidine has been reported to potentiate the anesthetic effect of local anesthetics and improve the quality of postoperative analgesia when used as an adjuvant in neuraxial block. We compared the effects of intravenous dexmedetomidine and midazolam for sedation on combined spinal-epidural (CSE) anesthesia.
Methods
This study included 50 patients undergoing total knee arthroplasty. CSE anesthesia was given using 10 mg bupivacaine for all patients. After checking the maximum sensory and motor levels, the patients were randomly allocated into two groups of 25 each to receive intravenous continuous infusion of dexmedetomidine (Group D) or midazolam (Group M) for sedation during surgery. Regression block level, hemodynamic changes, and sedation score were compared between the groups when the patients entered the postanesthetic care unit (PACU). For patient-controlled epidural analgesia, 0.2% levobupivacaine with 650 µg of fentanyl (150 ml in total) was infused at a rate of 1 ml/h, in addition to a 3-ml bolus dose with a 30-min lockout time. The visual analogue scale scores, additional analgesic demand, patient satisfaction, and adverse events between the two groups were also compared postoperatively.
Results
A significant difference was observed in relation to the sensory block level in the PACU (Group D: 6.3 ± 2.1; Group M: 3.2 ± 1.9) (P = 0.002). The motor block level and other outcomes showed no significant intergroup differences.
Conclusions
Intravenous injection of dexmedetomidine, rather than midazolam, for procedural sedation is associated with prolonged sensory block, with comparable incidences of adverse events during CSE anesthesia.
INTRODUCTION
Combined spinal-epidural (CSE) anesthesia is widely used for total knee arthroplasty (TKA) because it can reduce the disadvantages of general and spinal or epidural anesthesia alone. Compared to general anesthesia, CSE anesthesia appears equally effective and entails a shorter hospital stay without increased morbidity [1]. CSE anesthesia also offers the speed of onset, efficacy, and minimal toxicity of spinal anesthesia and extends the analgesia into the postoperative period [2]. These advantages make CSE anesthesia useful for surgical anesthesia and postoperative pain control in TKA. Moreover, increasing evidence suggests the use of sedation with sedative/hypnotic drugs to ensure patient comfort; this includes the use of midazolam or dexmedetomidine, which are the most commonly used drugs during surgeries under CSE anesthesia [3].
Dexmedetomidine is a highly selective α2-adrenoceptor agonist with sedative, amnestic, sympatholytic, and analgesic properties [4]. Several recent studies have shown that dexmedetomidine could potentiate the anesthetic effect of local anesthetics and improve the quality of postoperative analgesia when used as an adjuvant through the perineural, intrathecal, epidural, or systemic (intravenous) route [5,6]. However, a literature search revealed no human clinical trials comparing the effects of an intravenous sedative dose of intraoperative dexmedetomidine and midazolam on CSE anesthesia and postoperative analgesia.
We hypothesized that a sedative dose of intravenous dexmedetomidine would enhance sensory and motor blocks under CSE anesthesia, as well as improve the quality of postoperative analgesia, than would a sedative dose of midazolam in patients undergoing TKA. We planned a prospective, double-blind study to compare the effects of an intraoperative infusion of dexmedetomidine and midazolam for sedation with respect to sensory and motor blocks, postoperative analgesia, additional analgesic demand, and adverse events in CSE anesthesia for TKA.
MATERIALS AND METHODS
Enrollment
This study was approved by the Institutional Review Board of our hospital (no. 05-2016-073) and was registered at the Clinical Research Information Service (http://cris.nih.go.kr). All participants provided written informed consent. The study included 50 consecutive patients with American Society of Anesthesiologists physical status 1 to 3, aged under 85 years, and scheduled to undergo TKA. Patients with contraindications to neuraxial block (e.g., coagulation defects, infection at the puncture site, or pre-existing neurological deficits in the lower extremities), sensitivity to the study drugs, and intellectual impairments or psychiatric conditions precluding adequate communication were excluded from the study.
Study design
In the operating room, standard monitors were applied to the patients, and they were preloaded with 10 ml/kg body weight of intravenous crystalloid solutions over 15–20 min. Oxygen was provided via a nasal cannula at the rate of 3 L/min. Patients were placed in the lateral decubitus position for CSE anesthesia. The CSE anesthesia was performed by a single anesthesiologist. Under aseptic precautions and 2% lidocaine skin infiltration, anesthetic block was performed through the midline approach in the L3–L4 or L4–L5 intervertebral space. The lumbar epidural space was identified using an 18-gauge Tuohy needle included in the spinal–epidural combined needle kit (CombiSpeed®, Ace Medical, Korea) by using the loss of resistance to saline technique; thereafter, an extralong 27-gauge pencil-point spinal needle was introduced through the Tuohy needle. When correct placement of the spinal needle was confirmed by the free flow of cerebrospinal fluid, 2.0 ml of 0.5% hyperbaric bupivacaine was injected. After withdrawing the spinal needle, an epidural catheter was introduced, about 3–4 cm into the epidural space through the Tuohy needle for postoperative analgesia. After confirming the proper regional anesthesia level and achieving hemodynamic stability, a list of random numbers generated using Excel (Microsoft Corporation, USA) was used to randomly assign patients into 2 groups (D and M): Group D (n = 25) received a loading dose of 1 µg/kg of intravenous dexmedetomidine (Precedex®, Hospira, USA) via an infusion pump over 10 min followed by a maintenance dose 0.1–0.5 µg/kg/h, and Group M (n = 25) received a loading dose of 0.05 mg/kg of intravenous midazolam and a maintenance dose of 0.03–0.06 mg/kg/h. The sedative drugs (dexmedetomidine and midazolam) were prepared in unlabelled 50 ml syringes as the same volume and were recorded separately by a co-investigator who was not involved in this study. Also this co-investigator, who prepared the drugs, attended to the patients and performed anesthetic care during the operation. Sedation was maintained at the level of Ramsay sedation scale score ≥ 3 (Ramsay sedation scale: 1 = patient anxious, agitated, or restless; 2 = patient cooperative, oriented, and tranquil alert; 3 = patient responds to commands; 4 = asleep, but with brisk response to light glabellar tap or loud auditory stimulus; 5 = asleep, sluggish response to light glabellar tap or loud auditory stimulus; and 6 = asleep, no response) [7]. The sedation state was assessed every 5 min to titrate the rate of drug infusion during the operation. Continuous infusion of these sedative drugs was discontinued when subcutaneous suturing was started and total drug infusion time was recorded. A blinded investigator, who was not directly involved in the anesthetic care of the patients, collected all study data. To eliminate any possible effects of surgical technique, all surgeries were undertaken by a single experienced orthopedic surgeon using the same surgical technique and prosthesis type in all patients. Patients and the orthopedic surgery team were blinded to group allocation.
For postoperative patient-controlled epidural analgesia, 285 mg of 0.75% levobupivacaine, 650 µg of fentanyl, and 99 ml of normal saline (150 ml in total) was started after wound closure and continued for 72 h (1 ml/h basal, 3 ml bolus, and 30 min lockout) by using an elastomeric infusion pump (Anaplus®, Ewha Meditech, Korea).
In the operating room, hemodynamic parameters (heart rate, non-invasive blood pressure) were checked every 5 min. Hypotension (defined by a decrease in mean arterial pressure below 20% of baseline or systolic pressure < 90 mmHg) was treated with intravenous ephedrine 5 mg and additional lactated Ringer’s solution (200 ml over a 5 min period). Bradycardia (heart rate < 45 beats/min) was treated with intravenous atropine 0.5 mg.
When the patients entered the postanesthetic care unit (PACU) after the completion of the surgery, their hemodynamic parameters and sensory and motor block levels were assessed and recorded immediately. If a patient requested additional pain control, 25 to 50 mg of pethidine was administered intramuscularly. Adverse events including dizziness, nausea, vomiting, epidural catheter-induced complications, and other neurologic deficits were noted.
Outcome measurements
The primary outcome was the degree of regression level of sensory block. Immediately after CSE anesthesia, the level of maximal sensory block was assessed with the patients in the supine position by using pinpricks with a 25‐gauge 5/8‐inch needle (Profi Needle, Shinchang Medical Co. Ltd., Korea). The maximal sensory level was checked every 2 min until the highest level had stabilized for four consecutive tests. When the patients entered the PACU after the completion of the surgery, the sensory level at the PACU was also assessed same methods.
Motor block was assessed immediately after the sensory check by using a modified Bromage scale (0 = no paralysis; 1 = unable to raise the extended leg; 2 = unable to flex the knee; and 3 = unable to flex the ankle) [8].
The level of sedation was evaluated after 30 min of continuous infusion of the sedative drugs and immediately after admission to the PACU by using the Ramsay sedation scale. Excessive sedation was defined as a score greater than 5/6 in the PACU.
The severity of postoperative pain was assessed and monitored using a visual analogue scale (VAS) immediately after admission to and discharge from the PACU, and at 1, 6, 24, 48, and 72 h postoperatively. If a patient requested additional pain control, 25 to 50 mg of pethidine was administered intramuscularly. Additional analgesic demand was registered. Adverse events including dizziness, nausea, vomiting, epidural catheter-induced complications, and other neurologic deficits were noted. Patient satisfaction regarding anesthetic care was assessed on a four point Likert scale as follows: 4 = very satisfied; 3 = somewhat satisfied; 2 = somewhat dissatisfied; and 1 = very dissatisfied [9].
Sample size estimation
We have reviewed several studies to know sensory block prolongation of dexmedetomidine after CSE anesthesia, and there was no study of how sensory levels are maintained immediately after surgery at the PACU. There was a study that measured the regression time of the sensory level, so we had to calculate the sample size through the study [10]. For this, we assumed a standard deviation (SD) of 56 min in time to sensory regression of two dermatomes, a type I (α) error of 0.05, and a type II (β) error of 0.2. To show a 20% difference in sensory regression of two dermatomes, at least 20 patients per group were needed. Considering potential drop-outs, we decided to enroll 25 patients in each group for the study.
Statistical analysis
All statistical analyses were performed using PASW Statistics for Windows/Macintosh, Version 18.0 (SPSS Inc., USA). Categorical data were presented as numbers and percentages, while continuous data were presented as means ± SDs. Student’s t-test was used to compare between two groups of continuous data, and intergroup differences in nonparametric variables were compared using the Mann–Whitney U test. Categorical data were compared using chi-square tests. A P value < 0.05 was considered statistically significant.
RESULTS
Fifty patients were enrolled in this study. One patient in Group M accidentally pulled out the epidural catheter within the first 24 h after the surgery, and was hence exclude from the study; the remaining 49 patients completed the study (Fig. 1). With respect to demographic data, no differences were observed in sex, American Society of Anesthesiologists physical status, age, height, weight, anesthesia time, total infusion time of sedative drugs, and PACU stay time between the two groups (Table 1).
As the baseline value, the maximal sensory level increased from the needle insertion site to 7.7 ± 2.2 in Group D and to 6.8 ± 2.2 in Group M, which was not significantly different between the two groups. The sensory block levels in the PACU were 6.3 ± 2.1 in Group D and 3.2 ± 1.9 in Group M. The sensory block level in the PACU was significantly higher in Group D than in Group M (P = 0.002) (Table 2). However, no significant difference was observed between the two groups in the maximal motor block scores and motor block scores at the PACU determined using the modified Bromage scale (Table 3). The Ramsay sedation scale scores after 30 min of continuous infusion of the sedative drugs and immediately after admission to the PACU were not different in both the groups (Table 4).

The Maximal Motor Block Scores and Regression Motor Block Scores at the PACU Determined Using a Modified Bromage Scale

Ramsay Sedation Scales after 30 Minutes of Continuous Infusion of Sedative Drugs and Immediately after Admission to the PACU
The postoperative VAS scores immediately after admission to and discharge from the PACU, and at 1, 6, 24, 48, and 72 h after the surgery were 0.4 ± 2.0, 3.2 ± 7.5, 22.0 ± 8.2, 28.4 ± 6.9, 19.2 ± 10.4, 24.8 ± 7.3, and 27.1 ± 5.9, respectively, for Group D, and 0.8 ± 2.8, 2.1 ± 6.6, 22.9 ± 8.6, 28.3 ± 6.4, 22.9 ± 10.0, 22.5 ± 9.4, and 26.3 ± 6.0, respectively, for Group M. No significant intergroup differences were observed in the VAS scores immediately after admission to and discharge from the PACU, and at 1, 6, 24, 48, and 72 h after the surgery (Fig. 2). During the postoperative period, additional analgesic demand was not significantly different between the two groups. Patient satisfaction with postoperative pain control was also not significantly different between the two groups (Table 5).
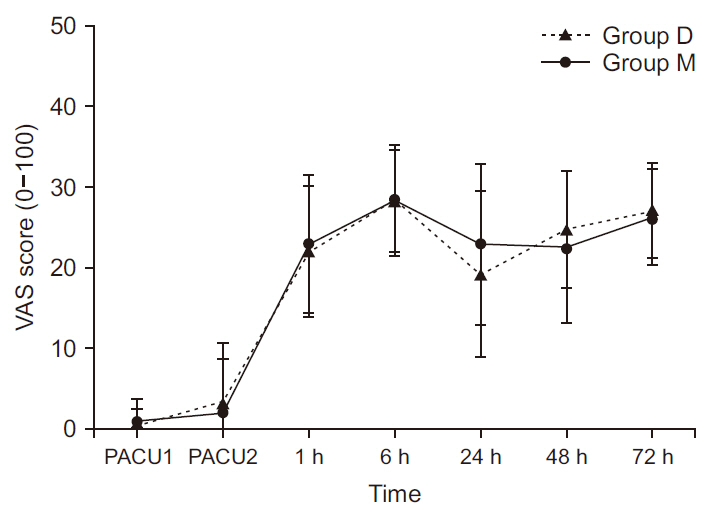
Changes in the visual analogue scale (VAS) score immediately after admission to and discharge from the PACU, and at 1, 6, 24, 48, and 72 h after surgery. All measured values are presented as mean ± standard deviation. PACU: postanesthetic care unit, PACU1: immediately after admission to the PACU, PACU2: at discharge from the PACU.
Regarding adverse events, 2 patients (8.0%) had nausea and 1 patient (4.0%) had dizziness in Group D, while 1 patient (4.2%) had nausea and 1 patient (4.2%) had oozing at the catheter insertion site in Group M. In hemodynamic changes during the operation, bradycardia and hypotension were occurred 1 patient (4.0%) and 4 patients (16.0%) in group D, and 1 patient (4.2%) and 2 patients (8.3%) in group M, respectively. There were no statistically differences in both groups. No other adverse events attributable to the drugs and procedure were noted.
DISCUSSION
In this randomized, double-blind, comparative study, we explored the potential benefits of intravenous dexmedetomidine for procedural sedation during TKA on CSE anesthesia and postoperative analgesia. Our findings showed that intravenous dexmedetomidine, rather than midazolam, for sedation was associated with prolonged sensory block during CSE anesthesia.
Dexmedetomidine is a potent α2-adrenoreceptor agonist with sedative and analgesic properties that have led to reductions in analgesic and anesthetic requirements. It has been most frequently used to sedate patients in intensive care unit settings and undergoing procedures. More recently, its off-label use in the absence of US Food and Drug Administration approval as a local anesthetic adjuvant has been increasingly reported to prolong the duration of anesthesia produced by single-injection neuraxial and peripheral nerve blocks [11,12]. Furthermore, several recent studies have shown that systemic (intravenous) and intrathecal administration of dexmedetomidine could similarly potentiate the anesthetic effect of local anesthetics. Thereby intravenous dexmedetomidine prolonged the duration of sensory block in spinal anesthesia with low-dose bupivacaine, decreased the requirement of supplemental analgesics, and improved the quality of postoperative analgesia [13–15].
In our study, to avoid potential risks pertaining to the “off-label” use of dexmedetomidine, we compared sedative doses of intravenous dexmedetomidine and midazolam, which is another commonly used intravenous sedative, during surgery under regional anesthesia. We observed no difference between the groups in the maximal sensory level before the administration of the drugs; however, after intraoperative administration of sedatives, the sensory block level in the PACU was higher in the dexmedetomidine group than in the midazolam group. These findings are similar to the results of the previous studies. Although this study showed that intravenous dexmedetomidine prolonged the duration of sensory block, the underlying mechanism is not fully understood. One study demonstrated that dexmedetomidine has an inhibitory effect on the locus ceruleus (A6 group) and dorsal raphe nucleus, which is located at the brain stem [16]. Besides, according other studies, not only this supraspinal action but peripheral vasoconstricting action could explain the prolongation of spinal anesthesia after intravenous administration of dexmedetomidine [17,18].
Accumulating data suggest that intravenous dexmedetomidine selectively prolonged the duration of sensory block without prolonging motor block, whereas perineural or intrathecal dexmedetomidine significantly prolonged the duration of motor block [19]. Another study showed that intravenous dexmedetomidine prolonged motor block as well as sensory block in spinal anesthesia, but the prolongation of motor block was less than that of sensory block [13]. In agreement to these findings, our findings showed that, compared with the prolongation of sensory block, the motor block level in the PACU was not significantly different between the study groups. Although the mechanism of motor block is not yet clear, this result could be explained by that the conduction of sensory nerve fibers might be more inhibited than that of motor nerve fibers at the same concentration of dexmedetomidine, as similarly reported with the use of clonidine [20]. Some evidence suggests that clonidine results in the direct inhibition of impulse conduction in the large, myelinated Aα fibers, with a 50% effective concentration (EC50) approximately four-fold higher than that in small, unmyelinated C fibers. The same mechanism might be applied to the action of dexmedetomidine, and would explain the greater sensory rather than motor block prolongation. However, these findings provide limited evidence to draw conclusions regarding the effect of intravenous dexmedetomidine on motor block because of the potential influence of several confounding factors. In addition, the results of the present study contradict those of a systematic review [21], which suggested that motor block with spinal anesthesia is prolonged when intravenous dexmedetomidine is administered. However, that review article had several limitations, including the limited number of small trials included and the lack of standardized assessment of motor block. In our study, motor block was assessed only once in the PACU, and it still persisted in both the groups. Moreover, the dose used in our study was lower than those used in previous studies of that review article.
Several studies have reported that postoperative pain was reduced during the postoperative period when dexmedetomidine was administered intraoperatively in regional anesthesia [21,22]. These results are explained by that dexmedetomidine has a role in modulating pain and inhibiting the transmission and perception of pain [23]. Contrary to the above studies, the present study reported no significant difference in postoperative VAS score and additional analgesic demand between the two groups. However, the VAS score and additional analgesic demand throughout the 72-h postoperative period were low in all patients in both the groups. This may be explained by our postoperative pain management regimen using patient-controlled epidural analgesia could provide adequate analgesia.
There are several considerations regarding the use of such sedatives. The most common adverse effects of dexmedetomidine are bradycardia and hypotension [24]. Another consideration is the prolonged recovery time from sedation after dexmedetomidine infusion. Dexmedetomidine does not cause respiratory depression, whereas midazolam is known to cause apnea and arterial desaturation [25,26]. Nevertheless, no serious adverse events were noted in our study and the satisfaction scores were high in both the groups. As a result, the optimal sedation was achieved with minimal adverse events.
This study has several limitations. First, it did not account for the duration of motor block. We limited our observations to sensory block characteristics because the primary aim of the study was to identify whether dexmedetomidine or midazolam was more efficient in providing a longer pain-free period. Second, we could not establish a normal saline control group, because proper sedation had to be maintained during the surgery. In addition, since the patient is in a sedated state during the surgery, the sensory and motor block levels should be assessed only in the PACU, and not during the surgery. The third limitation, the dose used in our study was that common using clinically, and the dose of dexmedetomidine might not be equipotent to that of midazolam; we suggest further studies to determine the equipotential dose ratio of dexmedetomidine to midazolam.
In conclusion, we found that the use of intravenous dexmedetomidine, rather than midazolam, for procedural sedation in patients undergoing TKA might prolong sensory block during CSE anesthesia and provide proper sedation with comparable incidences of adverse events. Thus, dexmedetomidine sedation may be preferable to midazolam sedation if prolonged operation time is expected to require a prolongation of sensory block.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.




