Suspected transcutaneous cerebral spinal fluid leakage without postural headache after implantable intrathecal drug delivery system removal - A case report -
Article information
Abstract
A 55-year-old man with an implantable intrathecal drug delivery system (IDDS) implant removal surgery was performed to control a suspected implant infection. Clear discharge from a lumbar wound was detected after IDDS removal, but transcutaneous cerebral spinal fluid (CSF) leakage was not suspected because the patient did not suffer from a postural headache. Finally, a suspected CSF leakage was resolved with a single epidural blood patch.
An implantable intrathecal drug delivery system (IDDS) could be used for various malignant or non-malignant chronic pain [1]. Even though the IDDS insertion is an invasive procedure that makes a direct conduit into the central nervous system for analgesics delivery, the use of IDDS is increasingly popular because it is very effective in treating chronic intractable pain and is supported by multiple studies [2,3]. We report on the case of a patient who had a clear discharge from a suspected cerebral spinal fluid (CSF) leakage through a lumbar wound after intrathecal morphine pump removal surgery. It was an uncertain case of CSF leakage because the patient did not suffer from a headache. Finally, the clear discharge was successfully resolved by a single epidural blood patch.
CASE REPORT
A 55 year-old man had a post herpetic neuralgia (PHN) affecting the right shoulder and arm for 10 years. He experienced piercing, severe burning pain (visual analogue scale [VAS] 8/10) that was not easily managed by various medications such as oxycodone, morphine, fentanyl transdermal patch, milnacipran, and pregabalin. Pain interventions such as stellate ganglion block, cervical epidural block, and brachial plexus block were performed multiple times. Those procedures only relieved his pain for a few brief moments.
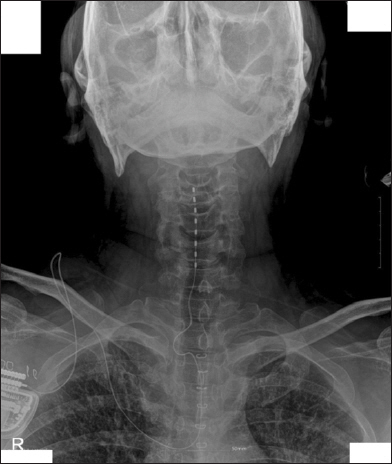
Eight years ago, a spinal cord stimulator was implanted on his cervical spinal cord area though it did not successfully relieve the pain (Fig. 1). An intrathecal morphine pump insertion was performed to control the intractable PHN. Other physical examination and laboratory results was unremarkable. His coagulation profiles were within the normal ranges.
In the intrathecal morphine trial, his right arm pain was reduced to VAS 5/10 with the injection of 0.3 mg morphine sulfate into the L3–4 interspinous space. A permanent morphine pump was implanted two days later.
The morphine pump implantation was conducted under general anesthesia. On the right decubitus position, 14-gauge tuohy needle was inserted through a 2 centimeter lumbar incision at the right L4 region. A catheter was inserted into intrathecal area through a tuohy needle at L2. An intrathecal catheter was fixed at the body at T3. The infusion pump was implanted on the subcutaneous layer of right anterior suprailiac region. There was no specific event during implantation surgery (Fig. 2).
One gram of intravenous cefazolin was injected every day for a week. The patient was discharged eight days after the implantation surgery without any issue.
Three weeks after his discharge he came to our pain management clinic for sudden sensitivity and tenderness on the area surrounding the abdominal morphine infuser. The abdominal wound seemed clean without any discharge or feverish feeling to the touch. There was tenderness just above the morphine pump. The patient was admitted into our hospital and blood tests were performed. Body temperature was 36.6°C and the C-reactive protein (CRP) was elevated to 25.5 mg/L (Fig. 3).

Laboratory value trends after morphine pump removal. hsCRP: high-sensitivity C-reactive protein, POD: postoperative day.
He received intravenous vancomycin of 1 gram, two times a day, and aztreonam 2 grams, three times a day, was started to control a suspected infection. The body temperature was kept around 36.6°C, but CRP was elevated to 64.1 mg/L. On the third day in the hospital, oral metronidazole 500 mg was added to his medication. Eventually he required emergency implantation removal surgery under local anesthesia on the sixth hospital day, because his body temperature and heart rate increased to 39.6°C and 119 per minute. The abdominal wound was aggressively irrigated with vancomycin and closed primarily because no abscess or dirty discharge was observed during removal surgery. A wound culture was performed, but no organism grew on the culture medium.
On the first postoperative day (POD 1) he complained of a continuous discharge from the lumbar wound (Fig. 4). The discharge was clean, odorless, and determined to be serious. His body temperature dropped to 36.6°C.
He did not have specific symptoms such as chill, headache, hot to the touch on the wound. Vital signs were within normal range, but the CRP was elevated to 59.2 mg/L on POD 3. On POD 4, lumbar wound infection was suspected. The lumbar wound was explored under local anesthesia. The wound was clean and pinkish. There was no sign of infection. On POD 4, though the patient did not complain of headache, CSF leakage through the lumbar wound was suspected. An epidural blood patch was used to diagnose CSF leakage. Fifteen milliliters of autologous blood was injected into the epidural space between L2 and L3.
The lumbar discharge was stopped just after the epidural blood patch. On POD 6, CRP was dropped to 17.1 mg/L. The wound healed without any specific event. Intravenous vancomycin in the amount of 1 gram, two times a day, was injected until POD 7. Wound stitches were removed at POD 10 without any problem.
DISCUSSION
IDDS can be applied when pain cannot be resolved with conservative managements such as physical therapy, psychological and behavioral intervention, pharmacotherapy, and minimally invasive intervention such as epidural steroid injection [4]. Coagulation problems and infections should be controlled for and an intrathecal drug trial is strongly recommended prior to implantation surgery [5].
Intra-operative bleeding can lead to a pump site hematoma, epidural, intrathecal, or subdural hematoma. Anti-coagulation guidelines for intra spinal therapy should be strictly followed [6].
Catheter related complications are the major cause of failure in IDDS drug delivery. It was shown that 5–6% of IDDS patients had a migration or fracture of the catheter that caused IDDS failure [7].
IDDS is an implanted foreign body and is susceptible to infection. Strict sterile techniques and preventive antibiotics are required to prevent infections. Cephalosporin and vancomycin are frequently used because most infections are caused by staphylococcus aureus [8]. It is not necessary to treat all wound infections to remove the implant, but suspicious wounds should be cultured. If the catheter or implant pocket is involved in the infection, then all implant hardware should be removed immediately. After implant removal, aggressive antibiotic therapy should be started [4]. The patients with IDDS implants should be carefully monitored for infection, since there was a report that patients with chronic neuropathic pain had more significant epidural infections than patients with somatic pain [9].
In this case, the patient had a long intrathecal catheter that reached to the T3 body. It seems likely that a long catheter and prolonged operation time to handle the catheter might be somewhat responsible for the suspected wound infection.
Kurnutala et al. [10] reported on a transcutaneous CSF leakage case with persistent spinal headache after removal of IDDS that was successfully managed with a surgical dura repair. Their patient had a postural headache with CSF leakage through an open paraspinal surgical wound. They chose surgical correction of transcutaneous CSF leakage which is an sign that urgent surgery is needed [1].
In this case, the patient had clear discharge at the lumbar wound after implant removal. CSF leakage was not suspected at first because the patient did not have a headache. Most patients with CSF leakage had a orthostatic postural headache. It worsened with sitting and was relieved with horizontal positioning [10]. We were concerned that the clear discharge was related to the wound infection in the lumbar wound because the CRP level did not drop after implant removal. We realized the possibility of wound infection was low. Though CRP was kept high the patient had no fever. No feverish sense or tenderness was detected on the lumbar wound. When the lumbar wound was explored in the operation room the wound was clean and no sign of infection was observed. On POD 4, CSF leakage was finally suspected. It was not certain that the clear discharge was CSF. There were no clinical symptoms such as postural headache supporting CSF leakage. It was difficult to confirm the CSF leakage by lab tests for CSF because it was not possible to collect enough of it that was a clean discharge for the CSF lab test.
CSF leakage was strongly suspected though our team hesitated to get into urgent surgical exploration. It seemed likely that surgical exploration was too invasive a procedure for this patient. Epidural blood patch was chosen to diagnose CSF leakage. A single epidural blood patch was useful to diagnose and resolve the clear discharge.
Hypotension in subarachnoid spaces inducing grip or traction of dura, blood vessels, or cervical nerve roots was the suggested mechanism of headache from post dural puncture for a long time [11]. However, not all patients with CSF hypotension from dural puncture suffered from headaches [12]. Additionally, not all patients with post dural puncture headaches had CSF hypotension [13]. Dunbar and Katz [14] reported post dural puncture could lead thoracic pain without headache. They suggested that post dural puncture can lead to thoracic pain without headache by grip or traction of spinal cord roots.
Our patient might have had an arm or shoulder pain with CSF leakage without headache, but he did not complain of that much pain. This may be because he might have confused some post herpetic neuralgia on his upper extremity.
It is not clear why the CRP level was elevated without infection in CSF leakage. It might be possible that transcutaneous CSF fistula could hinder the healing of a surgical wound.
There were some limitations of this case study. First, CSF leakage could not be confirmed by laboratory tests. Second, if there was a hidden infection close to the epidural space an epidural blood patch could delay the early surgical intervention to prevent epidural abscess or meningitis.
In a recent retrospective study, 23% of the patients with IDDS suffered from a post-dural-puncture-headache, 79% of the patients recovered with conservative treatments such as bed rest, hydration, and medications (caffeine, theophylline, antiemetics, analgesics, and steroids) [10]. Interventional treatments such as epidural blood or fibrin glue patches were required for 21% of the patients. Eighty-eight percent of interventional treatment groups were successfully treated by a single epidural blood patch [15].
In conclusion, orthostatic headache is the main symptom as evidence for CSF leakage from IDDS. Headache alone should not be used as the only key or critical symptom to diagnose CSF leakage when clear discharge on the lumbar wound is observed after implant removal. Epidural blood patch could be a useful tool to resolve and diagnose transcutaneous CSF leakage if a surgical wound infection can be excluded.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
ORCID
Jaeyoung Yang: https://orcid.org/0000-0003-1370-6646
Yusun Choi: https://orcid.org/0000-0002-0850-820X
Juyoung Park: https://orcid.org/0000-0002-2301-8057
Junhyug Jeong: https://orcid.org/0000-0002-6518-2170
Bousung Lee: https://orcid.org/0000-0001-5661-0922
Kwanghaeng Lee: https://orcid.org/0000-0003-1195-3184
Jaedo Lee: https://orcid.org/0000-0002-9416-5716