The effects of hydromorphone on astrocytic responses in cerebral ischemia
Article information
Abstract
Background:
Ischemic insult during operation could cause ischemic-reperfusion injuries in brain and memory impairments. Total intravenous anesthesia (TIVA) is preferred in brain surgery to promote the use of motor evoked potential monitoring and the use of opioids is common in TIVA. However there were few studies about ischemic protective effect of opioids to astrocytes.
Methods:
We used astrocytes, which were derived from human brain. We divided groups by conditioning period; i) pre-culture, ii) post-culture, or iii) pre + post-culture. All groups were treated 100 nM hydromorphone. We measured reactive oxygen species (ROS) by flow cytometry with 2’,7’-dichloroflurorescin diacetate. Then ROS in astrocytes which treated by opioid receptor antagonist were measured after treating 100 nM hydromorphone.
Results:
ROS was reduced in hydromorphone treated group, as compared to the control group (only tert-butyl hydroperoxide [TBH] treated). There was no difference in pre-conditioned group and post-conditioned group. However, ROS was much more reduced in pre + post-conditioned group compared to pre-conditioned only or post-conditioned only group. Furthermore each selective μ-, δ- and κ-opioid receptor antagonists partially negated the effect of hydromorphone.
Conclusions:
This study provides evidence that hydromorphone has both preconditioning and postconditioning effects on TBH- induced oxidative stress. Furthermore we proved each μ-, δ- and κ-opioid receptor relates to protective mechanism of hydromorphone to astrocytes.
INTRODUCTION
In recent times, astrocytes are considered to represent a specialized functional unit with neurons, in addition to providing neuronal support. It is known that astrocyte dysfunction is a cause of cognitive disorder or short-term memory loss [1]. Ischemic shock during surgery could cause ischemic-reperfusion injuries in the brain and memory impairments [2]. Many studies have established that opioids demonstrate protective effects against ischemia/reperfusion in different types of cells [3,4]. However, there are few studies that discuss the protective effects of opioids on astrocytes in ischemic conditions. Although the opioids have demonstrated preconditioning effects on the astrocytes in the brain [5], it remains to be proved that they possess post-conditioning effects as well. On the other hand, recent studies showed the hypoxic post-conditioning effects on the astrocytes in cerebral ischemia [6].
At present, total intravenous anesthesia (TIVA) is the preferred technique of anesthesia in neurosurgery for motor evoked potential (MEP) monitoring [7,8] and the perioperative use of opioids is common in such settings. Therefore, it is meaningful to investigate whether opioids possess pre and post-conditioning effects on astrocytes. In this study, we tried to investigate whether hydromorphone, one of the opioids that is widely used during the perioperative period, has pre and post-conditioning effects on astrocytes. Furthermore we tried to figure out whether pre and postconditioning of hydromorphone has synergic effects or not.
MATERIALS AND METHODS
Chemicals: Hydromorphone was purchased from Hanapharm Co. (Seoul, Korea), tert-butyl hydroperoxide (TBH) and 2’,7’-dichloroflurorescin diacetate (DCF-DA) were purchased from Sigma Chemical Co. (St Louis, MO, USA), the anti-glial fibrillary acidic protein primary antibody and Alexa Fluor 488 goat anti-mouse antibody were purchased from Millipore Co. (Billerica, MA, USA) and Invitrogen Co. (Carlsbad, CA, USA) respectively, XTT (2,3-Bis2-methoxy-4-nitro-5-sulfophenyl- 2H-tetrazolium-5-carboxanilide inner salt, WelCount Cell Viability Assay kit) was purchased from WELLGNE Inco. (Dalseo-gu, Daegu, Republic of Korea) and the mountain solution was purchased from Vector Laboratories (Burilingame, CA, USA).
Astrocytes: The cells used in this study were human brain- derived progenitor astrocytes (Gibco, St. Louis, MO, USA).
Cell viability
Cell viability was measured by XTT. The astrocytes were cultured in a 96-well plate. The control and TBH (100 uM) and/or hydromorphone (100 nM) treated astrocytes were placed in a new DMEM (Dulbecco’s Modified Eagle Medium), containing a 5% XTT reaction mixture (50 : 1 = XTT solution: PMS [N-methyl dibenzopyrazine methyl sulfate] solution) and incubated in a CO2 incubator, at a temperature of 37°C for a period of 2 hours. The XTT assay generated formazan crystals, which were measured spectro-photometrically with an ELISA reader (690 nm).
Experimental protocol
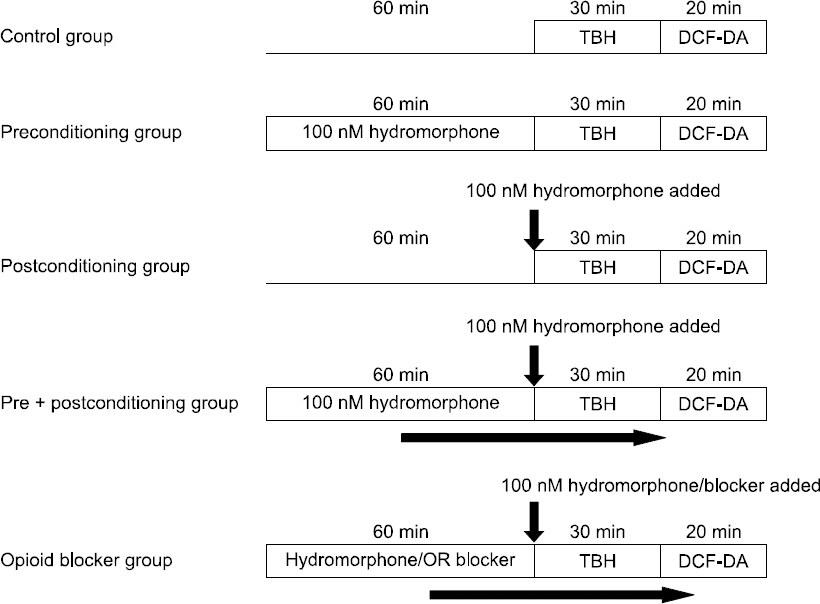
ROS-production induced by TBH was measured by flow cytometry using DCF-DA. We used the astrocytes for cell proliferation to 5 × 104 cells in each of the 6-well plates, and then transferred them to a serum-free medium, by performing overnight serum starvation. Thereafter, some of the cells were treated with hydromorphone during 3 different periods: i) pre-culture period (treating the cells with 100 nM hydromorphone for 1 hour and removing before treating with TBH), ii) post-culture period (co-treating the cells with 100 nM hydromorphone and TBH), and iii) pre + post-culture (treating the cells with 100 nM hydromorphone for 1 hour followed by co- treatment with 100 nM hydromorphone and TBH). The remaining cells were pre-treated for 1 hour with 100 nM hydromorphone and each of the selective μ-, δ- and κ-opioid receptor antagonists (naltrindole, nor-binaltorphimine, or D-Phe-Cys- Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 [CTOP]). The cells were treated with an opioid receptor antagonist followed by 100 nM hydromorphone and TBH and the intracellular ROS levels were measured by flow cytometry (Fig. 1).
The measurement of the intracellular ROS production
Flow cytometry: The control and TBH (50 μM) and/or hydromorphone (100 nM) treated astrocytes were incubated in a CO2 incubator with DCF-DA (10 ug/ml), at a temperature of 37°C for a period of 20 minutes. The DCF-DA-treated astrocytes were washed with phosphate-buffered saline (PBS) and detached from the plate by using trypsin-EDTA (0.25%). The detached cells were diluted with PBS (300 ul) for flow cytometric analysis. At least 3 different sets of experiments, with the cells from different isolations, were performed.
RESULTS
The effects of TBH and/or hydromorphone on ROS production
Production of ROS was lower in the hydromorphone-treated group as compared to the control, which was only TBH-treated, though the differences were not statistically significant. However, the ROS production was significantly lower in the pre + post-conditioned group compared to the preconditioned or the post-conditioned group (Fig. 2).
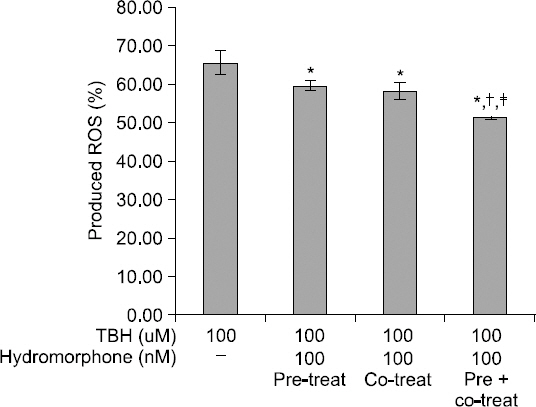
Detection of intracellular ROS. Detection of intracellular ROS products. This graph shows the protective effect of hydromorphone on TBH induced toxicity in primary human glial cell cultures. The ROS level was measured with using FACS (DCF-DA) in the 100 nM hydromorphone i) pre-conditioned ii) post-conditioned iii) pre+post-conditioned primary human glial cells. *< 0.05 versus control group. †< 0.05 versus preconditioned group. ‡< 0.05 versus post-conditioned group.
The effects of opioid receptor antagonists on ROS production
Production of ROS was reduced in the hydromorphone- treated group. However, each of the selective μ-, δ- and κ- opioid receptor antagonists partially negated the effects of hydromorphone on the ROS production (Fig. 3).
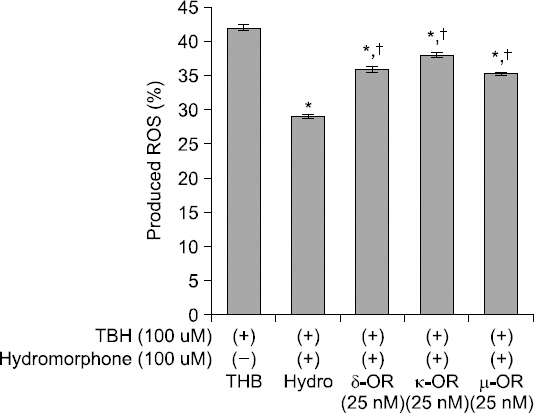
Detection of intracellular ROS. Effects of opioid receptor antagonists on ROS products. This graph shows that selective opioid receptor antagonists reverse the attenuation of the ROS induced by hydromorphone (pre + post-conditioned). The ROS level was measured with using FACS (DCF-DA) in the cells treated only TBH (control group), and the cells treated with TBH, hydromorphone and/or one of selective μ-, δ- and κ-opioid receptor antagonists (naltrindole, nor-binaltorphimine, and CTOP), respectively. *< 0.05 versus control group. †< 0.05 versus hydromorphone group.
DISCUSSION
At present, TIVA is the preferred technique of general anesthesia in cerebral surgery for MEP monitoring [7]. In addition, the use of opioids is common in TIVA for intraoperative analgesia. In this study, we demonstrated the preconditioning- and post-conditioning effects of hydromorphone. Therefore, our findings provide theoretical evidence of the perioperative effectiveness of hydromorphone.
Recent studies show that the opioids, including morphine, have a protective preconditioning effect in several ischemia/reperfusion models [9]. The anti-neuroinflammatory role and the prevention of memory deficit attributed to morphine, is due to its preconditioning effect, which is dependent on the opioid receptors [10]. However, the neuroprotective role of opioids in the case of astrocytes is unclear. Although, the glial cells play an important role in the antioxidant defense system in the brain [11], they are considered to be specialized functional units, in addition to providing physical support to the neurons. Astrocyte dysfunction is one of the causes of cognitive disorder or short-term memory loss [1]. In addition, neuroinflammation is highly related to ischemic stroke-induced cerebral damage and the progression of neurodegenerative diseases [12,13]. Astrocytes are important cells that are responsible for the neurophysiology of the brain and hence, represent potential therapeutic targets, as seen in this study.
Excessive oxidative stress is reported to be associated with a variety of diseases [14]. Although a certain ROS level is essential for cellular survival, cellular death may occur when oxidative stress caused by ROS production, exceeds the cellular antioxidant abilities [14,15]. An excessive ROS production has been reported to cause DNA damage, modify proteins and lipid functions, and activate the related signal pathways [15]. However, a moderate level of oxidative stress, referred to as positive oxidative stress, could be induced and modulated to produce an adaptive cellular response that is beneficial for cellular survival. Both, preconditioning and post-conditioning effects could be the result of positive oxidative stress [15,16]. Not only brief episodes of ischemia and reperfusion, but also a number of pharmacological agents can induce these phenomena [17]. TBH is commonly used to induce oxidative stress in-vitro and in-vivo [18]. In this study, we used TBH to induce oxidative stress-related injury to the murine-derived glial cells [19].
Certain studies demonstrated that morphine is involved in the functions of the immune system [20,21]. Morphine showed both, peripheral inflammatory and anti-inflammatory effects [22]. Although opioids induce glial activation and neuroinflammatory reactions [23], morphine-induced preconditioning showed an anti-neuroinflammatory effect [10].
Instead of morphine, we used hydromorphone, an opioid receptor agonist and a derivative of morphine [24], to prevent the TBH-induced ROS production. Hydromorphone, one of the first choice opioids as per the European guideline [25], is widely used in the management of acute or chronic cancer pain, in both, children and adults [25]. As compared to morphine, hydromorphone has certain advantages in clinical settings, such as lesser nausea and vomiting [26], and lower histamine release [27]. To demonstrate its protective effect, we used hydromorphone to reduce oxidative stress-related injury [28].
In this study, hydromorphone showed a protective effect towards TBH-induced oxidative stress-related injury, as assumed. Interestingly, the combined use of hydromorphone (preconditioning followed by co-administration) showed a superior effect when compared individually to preconditioning and co-administration treatments. It is known that the opioids not only suppress responses to various noxious stimuli (for example, short cyclic episodes of ischemia or ROS) but also show similar preconditioning effects [10,17]. This cumulative effect of hydromorphone may form an important basis in clinical settings, such as the perioperative uses of hydromorphone in neurosurgery.
Although most studies have accepted the role of δ-opioid receptors in ischemic models [3,9,29,30], the effects of μ- and κ-opioid receptors are still unclear. However, some studies have discussed the role of μ- and κ-opioid receptors in cardiac models [9,29]. It has been reported that the neuroprotective effects of hydromorphone, such as improved neurological score, reduced infarct volumes and tumor necrosis factor-α levels, are mediated through the δ-opioid receptors and not through the μ- and κ-opioid receptors, against transient focal cerebral ischemia in rats [30]. On the other hand, our results showed that each of the μ-, δ-, and κ-opioid receptors are involved in the protective effects of hydromorphone in astrocytes. When using each of the μ-, δ-, and κ-opioid receptor antagonists, the production of ROS is similar to that in the TBH-treated group. Even though we did not evaluate the mechanism of the opioid receptors in knock-out models, it gives possiblity to prove that each of the μ-, δ-, and κ-opioid receptors contribute to the protective effects of hydromorphone. Unfortunately, we cannot conduct these experiments with other opioid receptors, including toll-like receptor 4 (TLR-4), which is involved in the modulation by the glial cells [23]. Despite these shortcomings, our data suggest that regardless of the timing of administration, hydromorphone has protective effects, which corresponds to each of the μ-, δ-, and κ-opioid receptors. Hence, our findings support the therapeutic potential for hydromorphone in the prevention of ischemic cerebral injury.
In conclusion, this study provides evidence that hydromorphone has both, preconditioning and postconditioning effects on the TBH-induced, oxidative stress-related injuries. Furthermore, we proved that each of the μ-, δ-, and κ-opioid receptors contributes to the mechanism of hydromorphone and its protective effect on the astrocytes under ischemic conditions. Further studies are required to evaluate the mechanism of opioid receptors, including TLR-4, using knock-out models.