The effect of preemptive intravenous ketamine on postoperative pain in patients undergoing arthroscopic rotator cuff repair with intra articular ropivacaine injection
Article information
Abstract
Background:
A low dose of ketamine can be an effective preemptive analgesic by preventing central sensitization when administered before surgical trauma. In this study, we assessed the preemptive analgesic effect of low-dose ketamine administered intravenously to patients undergoing arthroscopic rotator cuff repair with intra articular ropivacaine injection.
Methods:
This randomized, double-blinded study included fifty-six patients scheduled for elective arthroscopic rotator cuff repair. Normal saline (group C) or 0.5 mg/kg of ketamine (group K) was injected intravenously before the skin incision. An intra articular injection using 20 ml of 0.75% ropivacaine was performed in both groups just before wound closure by the surgeon at the end of the surgery. Postoperative pain was assessed by the numeric rating scale (NRS) in the post-anesthesia care unit (PACU) and at 12, 24, and 48 hours postoperatively. The total dose of fentanyl consumption and side effects were recorded.
Results:
There were no significant differences between the C and K groups for the NRS of pain in the PACU and at 12, 24, and 48 hours after the surgery. In addition, there was also no significant difference in total fentanyl consumption between the two groups.
Conclusions:
Preemptive ketamine did not reduce preemptive pain scores and fentanyl consumption in patients who underwent arthroscopic rotator cuff repair with intra articular local anesthetic injection. Therefore, more aggressive and multimodal pain control is required in patients undergoing arthroscopic shoulder surgery regardless of the use of preemptive intravenous ketamine injection.
INTRODUCTION
Arthroscopic shoulder surgery is often associated with severe postoperative pain that can be difficult to manage without high-dose opioids [1,2]. However, high-dose opioids often result in side effects such as nausea, vomiting, urinary retention, and respiratory depression [3]. There are reports showing that supplementing a regional nerve block with intra articular local anesthetic injection (IA), interscalene brachial block (ISB), or suprascapular nerve block (SSB) can improve postoperative pain relief [1,2,4-6]. It has been reported that ISB is the most effective analgesic technique in arthroscopic shoulder surgery [7]; however, ISB is technically challenging and an invasive procedure compared to IA. On the other hand, IA is a simple and time conserving technique that can be performed during surgery but drawbacks include limited duration of action and less pain relieving effects than ISB and SSB, especially for single shot IA. An adjuvant for single shot IA is needed to prolong and enhance postoperative pain relief. There have been a few studies on the effect of ketamine as an adjuvant when a block is performed [8-10].
Ketamine, a non-competitive antagonist of the N-methyl-D- aspartate (NMDA) receptor, blocks nociceptive input and reduces hyperalgesia in low doses [11-14]. There are many studies on the use of ketamine in various types of surgeries, but there is controversy about its preemptive effect. There have been inconsistent results in previous studies [15] and there are few studies regarding the preemptive analgesic effect of ketamine in shoulder surgery.
Therefore, we designed this study to assess the efficacy of preemptive ketamine with intra articular ropivacaine injection in patients undergoing arthroscopic rotator cuff repair. We predicted a reduction in fentanyl consumption and opioid- related adverse effects due to the pain control effect of ketamine.
MATERIALS AND METHODS
This study was a prospective randomized double-blinded clinical trial. Before the operation, all patients enrolled in this study gave informed consent. The double-blind technique was used; therefore, the results of random allocation were not revealed to surgeons or assigned anesthesiologists. A total of 56 patients took part in this clinical trial. The patients ranged in age from 20 to 70 years old, had American Society of Anesthesiologists class I or II status, and were scheduled for elective arthroscopic rotator cuff repair. None of the patients had abnormal results on preoperative examinations. In addition, patients with complicated medical histories including conditions such as cardiovascular disease, cerebral vascular disease, epilepsy, drug allergies, drug intolerances, or psychiatric disorders were excluded from the study.
All enrolled patients were injected with 0.2 mg of glycopyrrolate as a premedication before induction and general anesthesia was induced with 4–6 mg/kg of thiopental sodium and 0.8 mg/kg of rocuronium. General anesthesia was maintained with sevoflurane and a gas mixture of 50% nitrous oxide and 50% oxygen.
The 56 patients were randomly assigned into two groups. Initial vital signs were recorded after monitoring for factors such as electrocardiograph, oxygen saturation, bispectral index score (BIS), and non-invasive blood pressure. After the patients were prepared for surgery by draping in the sitting position, we confirmed stable vital signs. Then, normal saline or ketamine was injected intravenously within 5 minutes before the first incision, with consideration for onset time. Patients in the control (C) group were injected with 5 ml of normal saline. Patients in the ketamine (K) group were injected with 0.5 mg/kg of ketamine diluted to a fixed volume of 5 ml. Blood pressure, heart rate, oxygen saturation, and BIS were recorded before the incision and at 5 and 10 minutes after the incision. An intra articular injection using 20 ml of 0.75% ropivacaine (Ropiva®, Hanlim, Pharm, Seoul, Korea) was performed in both groups just before wound closure by the surgeon at the end of the surgery.
A physician who was blinded to the patients’ assignment evaluated pain intensity and the numeral rating scale (NRS; 0 = no pain and 10 = worst pain imaginable) was used to estimate postoperative pain. Postoperative pain intensity was measured and recorded in the post-anesthesia care unit (PACU) and at 12, 24, and 48 hours postoperatively. The total dose of fentanyl consumed as rescue analgesia was recorded. A fentanyl patch (Durogesic® DTrans® 12 μg/h, Janssen-Cilag, UK) was used routinely upon arrival to the ward from the PACU. If the patient desired analgesics, 0.5–1.0 μg/kg of intravenous fentanyl was used in the PACU and in the ward. Monitoring of complications from fentanyl such as constipation, nausea, vomiting, bladder dysfunction, and respiratory depression was conducted. Other complications such as hallucinations, delirium, and delusions, which can be caused by ketamine, were also monitored and recorded.
All statistical analyses were carried out using SPSS® (software version 21.0, IBM Corporation, New York, NY, USA). The power calculation based on the expected difference in pain scores was 20%. The minimum sample size required in each group with a significance criterion (α) of 0.05 and a statistical power (1-β) of 0.8 was 23. The NRS and total fentanyl consumption were analyzed using the Mann Whitney U test. The Student’s t-test was performed to compare continuous variables between the two groups. Discrete variables were analyzed using the Chi-square test. A P value less than 0.05 was considered statistically significant. Data are presented as the mean ± standard deviation unless otherwise noted.
RESULTS
A total of 56 patients scheduled for arthroscopic rotator cuff repair were enrolled in this study. Demographic data between the two groups showed no significant differences (Table 1).
The NRS pain scores in the PACU were 3.60 ± 1.62 vs. 4.59 ± 2.2 (P = 0.088) in the C and K groups, respectively. The NRS pain scores at 12, 24, and 48 hours after the surgery were similar in the C and K groups, with values of 3.26 ± 1.10 vs. 3.07 ± 1.16 (P = 0.346), 2.60 ± 1.19 vs. 2.17 ± 2.04 (P = 0.146), and 1.52 ± 1.09 vs. 1.10 ± 1.3 (P = 0.057) in the C and K groups, respectively (Fig. 1). In addition, there was also no significant difference in total fentanyl consumption between the two groups (0.629 ± 0.02 μg vs. 0.627 ± 0.02 μg; P = 0.782) (Table 2). The total fentanyl dose was calculated by adding any rescue fentanyl doses to the amount of fentanyl absorbed (12 μg/h) from the fentanyl patch in 48 hours. Four of the patients complained of nausea and vomiting. One of these patients was in group K and the other three patients were in group C. Also, one patient in group K complained of urinary retention. No other patients experienced side effects caused by ketamine (Table 2).
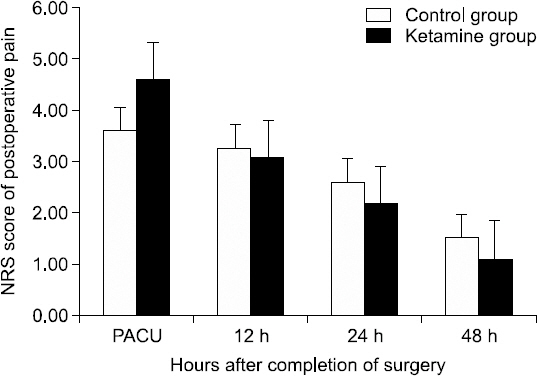
The above graph represents the pain intensity of the control and ketamine groups during the immediate 48 hours postoperative. Pain intensity was measured by a numeric rating scale (NRS; 0 = no pain and 10 = worst pain imaginable). There were no significant differences between the two groups (P > 0.05).
There were no significant differences in blood pressure, heart rate or BIS score before or after ketamine injection between the two groups (Table 3).
DISCUSSION
Although arthroscopic shoulder surgery is minimally invasive and commonly performed, it is often associated with severe postoperative pain [16]. Therefore, appropriate pain control can improve rehabilitation and recovery [17]. In our study, the mean NRS score was 3.6 in the PACU and the most severe NRS pain score was 10 in the PACU. For the relief of pain in shoulder surgery, opioids and nerve blocks can be used effectively. In our study, intra articular injections were administered to all patients for postoperative pain control. The surgeon performed IA by injecting 20 ml of 0.75% ropivacaine just before wound closure at the end of surgery. Some studies have demonstrated that single-dose IA ropivacaine reduces pain scores during the immediate postoperative period [4,18,19]. However, IA is less effective than interscalene block for postoperative pain relief [7]. Thus, for more effective postoperative pain control, we used preemptive ketamine.
It is well known that a high dose of ketamine can act as an anesthetic and a low dose can act as an analgesic [20]. A subanesthetic low dose of ketamine is defined as a single injection of less than 1 mg/kg or continuous injection of less than 0.1–0.2 mg/kg/h for pain management [20]. NMDA receptors play a significant role in spinal cord sensitization and chronic pain development after an acute injury. Activated NMDA receptors trigger pain pathways and contribute to events related to pain, such as wind-up phenomena or spinal neural plasticity [21]. Ketamine acts as a non-competitive antagonist for the phencyclidine receptor site on the NMDA receptor complex channel and can inhibit nociceptive input when bound to NMDA receptors [22]. Thus, ketamine prevents pain processing by NMDA receptors and reduces hyperalgesia. Injection of ketamine before noxious stimulus can block pain sensitization and eventually show a preemptive analgesic effect [11-13].
We used 0.5 mg/kg of ketamine and intra articular ropivacaine injection for pain relief in shoulder surgery. We expected decreases in pain scores and fentanyl consumption because we reasoned that both IA and preemptive ketamine injection would have strong pain relieving effects. However, there were no significant differences in NRS pain scores and fentanyl consumption in the PACU and at 12, 24, and 48 hours postoperatively between the two groups. Also, we believed that if ketamine has preemptive analgesic effects, there would be little effect on the BP, HR and BIS scores between the preincisional and postincisional periods. However, there were also no significant differences in vital signs and BIS scores. Preincisional administration of 0.5 mg/kg of ketamine failed to demonstrate sufficient preemptive analgesic effects in patients undergoing arthroscopic rotator cuff repair with intra articular ropivacaine injection.
Our results showed that the type of surgery could influence the effectiveness of preemptive ketamine. Several reports have reported varying results depending on the type of surgery. In gynecologic laparoscopic surgery, laparoscopic cholecystectomy and laparoscopic appendectomy, ketamine has preemptive analgesic effects, as seen during the early postoperative period [13,23,24]. In contrast, there are some reports showing that ketamine has no preemptive effect in cesarean sections and arthroscopic shoulder surgery [25,26]. The surgeries that are associated with preemptive ketamine effects are less invasive and, in contrast, more invasive surgeries are not associated with preemptive ketamine effects. The preemptive ketamine effect may be dependent on the intensity of the surgical pain. Arthroscopic shoulder surgery has continuous intense noxious stimuli throughout the procedure and postoperative period. Another possible contributing factor to the lack of preemptive ketamine effect may be inadequate ketamine dose. The dose of ketamine used in our study may be insufficient to block NMDA receptor activation in arthroscopic shoulder surgery. However, 0.5 mg/kg of ketamine (the dose used in our study) is a relatively high dose compared to doses used in other studies demonstrating the preemptive effects of ketamine and further study is needed to determine the optimal dose of ketamine in more painful surgeries.
Woo et al. [26] reported that ketamine has no preemptive analgesic effect in patients undergoing arthroscopic shoulder surgery despite a single-shot interscalene block. In this study, NRS scores were below three in both groups during the immediate 48 hours postoperative. However, because the interscalene block was performed before the induction, patients did not feel the incisional pain. So, preemptive ketamine effects could be masked by interscalene block. Unlike this study, we evaluated the effect of ketamine without a block of the incisional pain. Also, we controlled pain thereafter through the IA at the end of surgery.
There were several limitations of this study. First, we did not follow up on long-term outcomes. Second, because variability in the absorption rate from fentanyl patches in each patient was not considered, we could not measure the precise dose of fentanyl patch consumption. Third, results such as NRS and fentanyl consumption may be masked due to IA. However, we felt that controlling postoperative pain was of utmost importance because arthroscopic shoulder surgery is associated with very severe postoperative pain and IA was the routine procedure of the surgeon. Also, we designed this study for the purpose of evaluating the preemptive effects of ketamine in addition to IA performed at the end of surgery.
In conclusion, preincisional preemptive ketamine (0.5 mg/kg) did not reduce postoperative pain scores and fentanyl consumption in patients who underwent arthroscopic rotator cuff repair with IA local anesthetic injection. Therefore, aggressive and multimodal pain control is required in patients undergoing arthroscopic shoulder surgery, regardless of the use of intravenous preemptive ketamine injection as a preemptive analgesic. Further study is needed to determine whether a higher dose of ketamine would have preemptive effects in arthroscopic shoulder surgery.


