Mechanisms of postoperative pain
Article information
Abstract
Good pain control after surgery is important to facilitate overall recovery, improve patient satisfaction, decrease morbidity, and reduce health care cost. However, despite heightened awareness and development of new guidelines in recent decades, we have failed to make major improvements in postoperative pain control. Currently available analgesic therapies have limited efficacy, and pain after surgery continues to be a significant clinical problem. Our goal is to develop more effective and safer clinical strategies that will eliminate or greatly reduce postoperative pain, and a better understanding of the mechanisms of pain induced by surgery would be essential to achieve this goal. Evidence suggests that the pathophysiological mechanisms and optimal treatment of postoperative pain are different from many other painful conditions. Recognizing the necessity and importance of relevant pre-clinical models, we have developed and characterized rodent incision models that have close similarities to postoperative pain in patients. Previous studies have demonstrated the clinical relevance and translatability of these pre-clinical models of postoperative pain. In this review, we describe the rodent incision pain models, and summarized our current understanding of the mechanisms of postoperative pain, highlighting key findings from our previous studies using these models.
INTRODUCTION
Management of acute postoperative pain is an essential component of perioperative patient care. Good pain control after surgery is important to facilitate overall recovery, improve patient satisfaction, and reduce health care cost. Effective postoperative pain management is also likely to decrease morbidity and improve functional outcome. Moreover, severe acute postoperative pain is a risk factor for chronic postsurgical pain, raising more awareness regarding the importance of adequate perioperative pain management. Despite heightened awareness and clinical advancements in pain management, however, postoperative pain continues to be a significant clinical problem [1-3]; according to several recent reports and surveys, a significant percentage of patients (up to 70–75% for some types of surgery) experience moderate to severe pain while receiving analgesics during the first several days after surgery. These data indicate limited efficacy of the currently available analgesic therapies for postoperative pain. While recent guidelines support using multimodal regimens [4,5], opioids still remain the mainstay of perioperative pain management. However, dose titration of opioids is often limited by their tolerability and side effects such as respiratory depression, sedation, nausea, vomiting, pruritus, urinary retention, and constipation. Although peripheral and neuraxial regional techniques can provide an effective and valuable analgesic option, these techniques benefit only a small proportion of surgical patients. As the concept of multimodal analgesia became widely accepted over the past decade, non-opioid analgesics, such as non-steroid anti-inflammatory drugs (NSAIDs), acetaminophen, ketamine, and gabapentinoids, are more commonly used as part of a balanced analgesic approach. While we could achieve some success in improving quality of analgesia with concomitant attenuation of side effects through this approach, yet overall, we failed to make major improvements in postoperative pain control during the last couple of decades [1,3]. A better understanding of the mechanisms of pain induced by surgery would be essential for the development of more effective and safer clinical strategies that will eliminate or greatly reduce postoperative pain. Recognizing the necessity and importance of relevant pre-clinical models in studying the mechanisms of pain induced by surgery, we have developed and characterized rodent models that have close similarities to postoperative pain in patients [6-8]. In this review, we will first briefly describe these pre-clinical pain models, and then summarize our current understanding of the mechanisms of postoperative pain, highlighting key findings from our previous studies using these models.
PRE-CLINICAL MODELS OF POSTOPERATIVE PAIN
Plantar incision model [6,8]
1. Surgery (Fig. 1)
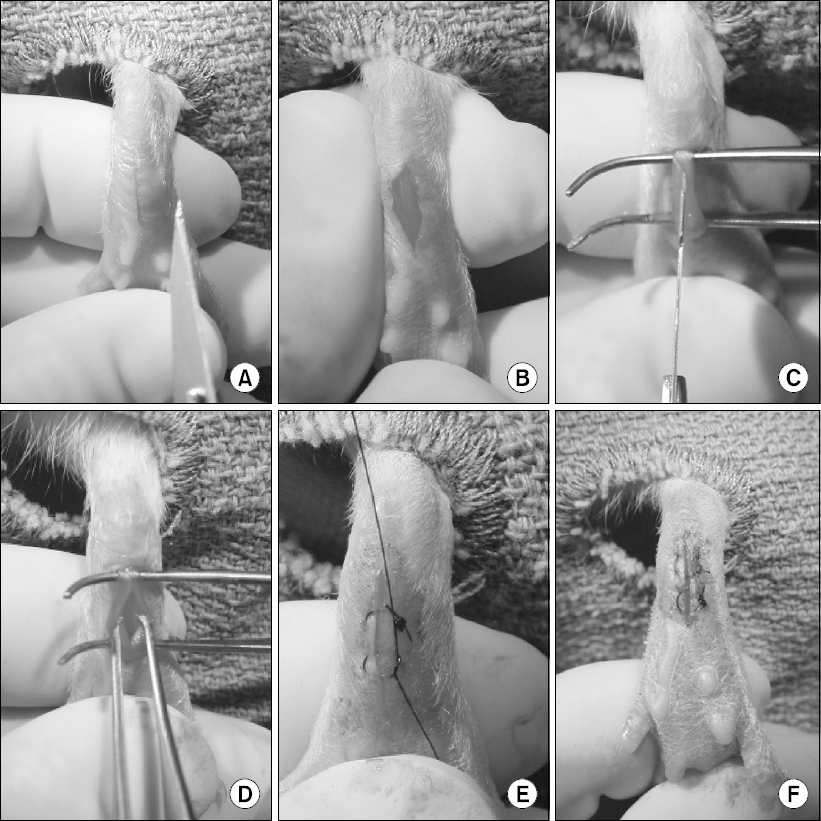
Rat plantar incision model. (A, B) A 1 cm longitudinal incision is made through skin and fascia, starting 0.5 cm from the proximal edge of the heel. (C, D) The flexor digitorum brevis muscle is elevated and incised longitudinally, with the muscle origin and insertion remained intact. (E, F) After hemostasis, the skin is closed with two mattress sutures.
Briefly, the rat is anesthetized and the plantar aspect of the hind paw is prepared in a sterile manner. A 1 cm longitudinal incision is made through skin and fascia, starting 0.5 cm from the proximal edge of the heel. The flexor digitorum brevis muscle is elevated and incised longitudinally, with the muscle origin and insertion remained intact. After hemostasis, the skin is closed with two mattress sutures. Similarly, a mouse plantar incision model also has been described [6,9].
2. Behavioral testing
The following pain-related behaviors can be measured after plantar incision: Non-evoked pain behaviors, such as guarding pain behaviors [10] and conditioned place preference (CPP) [11,12]; primary hyperalgesia, such as mechanical and heat hyperalgesia. Secondary hyperalgesia can also be evaluated after plantar hind paw incision.
1) Guarding pain behaviors [10]: A cumulative pain score can be used to assess non-evoked pain behaviors after plantar incision. Unrestrained rats are placed on a mesh floor (8 × 8 mm grid) and allowed to acclimate. The position of both paws are observed during 1 min period repeated every 5 min for 1 h. Depending of the position in which each paw is found during the majority of the 1 min period, a score of 0, 1, or 2 is given (Fig. 2). The sum of the twelve scores (0–24) recorded during 1 h session for each paw is obtained. The cumulative pain score is the difference between the scores from the incised and non-incised paw. Greater cumulative pain score would indicate greater guarding pain behavior. Typically, guarding behavior is the greatest immediately after incision, and gradually returns to pre-surgery baseline value over the next 4–5 days. We also have assessed guarding behaviors after plantar incision mice [6]. Various strains of mice exhibited guarding behavior for 2–3 days after incision, although the pain score during early postoperative period tended to be lower in mice (mean cumulative pain score 6–8) [6], compared to that in rats (mean cumulative pain score 15–24) [8,10,13]. Guarding behavior can be modified by clinically relevant dose of morphine or ketoprofen [10,14], suggesting that this pain-related behavior may be translatable to resting pain in patients after surgery.
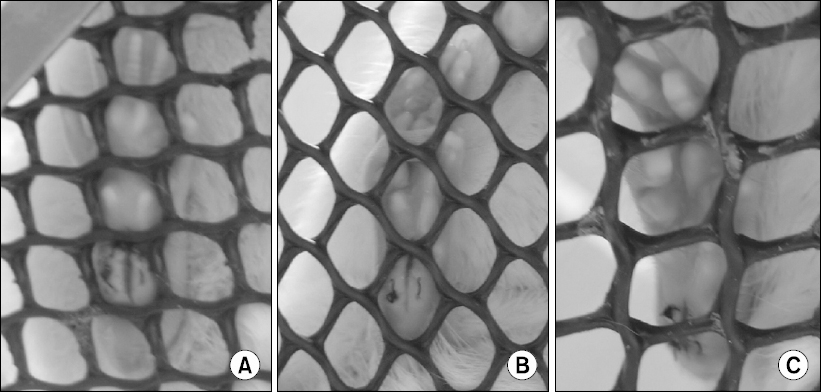
Guarding pain behavior after incision. The position of both paws are observed during 1 min period repeated every 5 min, and a score of 0, 1, or 2 is given, depending of the position in which each paw is found during the majority of the 1 min period; (A) A score of 0 is given for full weight bearing of the paw, causing blanching or distortion of the wound by the mesh; (B) A score of 1 is given if the area of the wound touches the mesh without blanching or distorting; (C) A score of 2 is given if the paw is completely off the mesh. The sum of the twelve scores recorded during 1 h session for each paw is obtained, and the cumulative pain score is the difference between the scores from the incised and non-incised paw.
2) CPP: To assess spontaneous pain behaviors after plantar incision, we have performed single-trial, biased CPP protocol using sciatic nerve block with bupivacaine [12]. In this approach, development of preference to the analgesia-paired chamber (initially non-preferred chamber) after conditioning would indicate presence of non-evoked, ongoing pain. The CPP apparatus consists of 2 larger end chambers and 1 smaller center-connecting chamber, and the 2 end chambers have differing visual, tactile, and olfactory cues. After 2 day handling session, baseline preference for chambers is measured with free access to all chambers, and the preferred and non-preferred chambers are identified. Rats spending greater than 80% or less than 20% time in one chamber are excluded from further testing. The rats then undergo plantar hind paw incision. In the morning of the conditioning day (postoperative day 1), vehicle treatment (e.g. sciatic nerve administration of phosphate-buffered saline) is performed, and the rats are placed into the preferred chamber for 45 min. In the afternoon, drug treatment (e.g. sciatic nerve block with 0.5% bupivacaine) is performed and rats are placed in the non-preferred chamber for 45 min. The following day (postoperative day 2), rats are placed in the CPP apparatus and time spent in each chamber is measured for 15 min. Development of preference for analgesia-paired chamber after hind paw incision has previously been demonstrated in a single-trial, non-biased CPP approach using lidocaine injection as well [11].
3) Mechanical hyperalgesia: Decreased withdrawal threshold to punctate mechanical stimulation at the wound has been characterized after plantar incision [8,15]. To assess mechanosensitivity, rats are place on a mesh floor (12 × 12 mm grid) and allowed to acclimate. Calibrated von Frey monofilaments are applied adjacent to the wound; each filament is applied once, starting with low force (e.g. 10 mN) then successively with higher forces, until the hindlimb withdrawal response is evoked or the filament with maximum force (e.g. 250–500 mN) is reached. This trial is repeated three times with at least a 10 min interval. The lowest force from three trials that produces a withdrawal response is considered the withdrawal threshold. Typically, the median withdrawal threshold decreases from the pre-surgical value (250–500 mN) to less than 100 mN for 2–3 days after incision, and gradually returns to the baseline by postoperative day 6–7. Primary mechanical hyperalgesia can also be seen after plantar incision in mice [6], and it follows a similar time course to that observed in rats.
4) Heat hyperalgesia: Decreased withdrawal latency to radiant heat stimulation to the wound has been characterized after plantar incision [15]. To assess heat sensitivity, animals are placed on a heat-tempered glass floor (3 mm thickness) and allowed to acclimate. A focused radiant heat source is applied from underneath the glass floor on the center of the incision. The intensity of the heat is adjusted to produce withdrawal latencies of 15–20 s in normal, un-incised animals. The latency to evoke paw withdrawal is measured to the nearest 0.1 s with a cutoff value of 30 s. This trial is repeated three times with at least a 10 min interval to obtain the average paw withdrawal latency. Typically, the mean withdrawal latency decreases from the pre-surgical value (10–12 s) to less than 3–5 s during early postoperative period, and gradually returns to the baseline by postoperative day 7–10. Primary heat hyperalgesia is also seen after hind paw incision in mice [6].
5) Secondary hyperalgesia, an exaggerated response to mechanical stimuli applied to undamaged area surrounding the site of incision, was observed after hind paw incision in rats [15]. Secondary hyperalgesia to punctate mechanical stimuli was rather short-lived, lasting 1–2 days after incision. Secondary hyperalgesia to heat stimuli was not observed in the plantar incision model.
Gastrocnemius muscle incision model
1. Surgery [7]
After shaving and sterile preparation, a 3 cm longitudinal incision is made through the skin of the posterior hindlimb. The cutaneous tissue is separated from the underlying muscle, and the fascia between the two bodies of the gastrocnemius muscle is split and divided using blunt dissection. After hemostasis, the skin is closed with four sutures.
2. Behavioral testing
We have previously reported persistent secondary hyperalgesia after gastrocnemius incision [7]; withdrawal thresholds to punctate mechanical stimuli, applied remote to the incised area, were reduced for about one week after incision. On the other hand, secondary hyperalgesia to heat stimuli was not observed in the gastrocnemius muscle incision model. Non-evoked, spontaneous pain behavior has not yet been thoroughly examined, although future investigation may be forthcoming.
SENSITIZATION OF THE SOMATOSENSORY PATHWAYS AFTER INCISION
Behavioral and neurophysiological studies using the pre-clinical incision models suggest that tissue injury caused by surgery results in both peripheral and central sensitization, contributing to postoperative pain. Sensitization of the somatosensory pathways is characterized by increased spontaneous activity, a left shift and an increase in slope of mechanical stimulus-response curve, and the expansion of the receptive field.
Peripheral sensitization
Tissue injury due to surgery causes the release of chemical mediators, resulting in peripheral sensitization at the level of the primary afferent fibers. We have demonstrated the presence of various pain mediators in the wound environment that can lead to activation and sensitization of the nociceptors [16-23]. Peripheral sensitization appears to be a major contributor to the ongoing pain and primary hyperalgesia after plantar incision [24-26].
1. Electrophysiological evidence for peripheral sensitization
1) Spontaneous activity: Peripheral sensitization could be manifested as ongoing, spontaneous activity of nociceptors in the absence of external stimuli. There is ample electrophysiological evidence demonstrating increased spontaneous activity of nociceptors after incision. When recorded in vivo in anesthetized rats on postoperative day 1, 39–61% of nociceptors innervating plantar incision exhibited spontaneous activity, whereas only 0–13% of nociceptors showed spontaneous activity in the sham surgery group [27,28]. Experiments using an in vitro preparation have demonstrated spontaneous activity of muscle and cutaneous nociceptors after incision as well [28-30]; while nociceptors innervating uninjured plantar flexor digitorum brevis muscle showed a low incidence (14%) of ongoing activity, 65% of group IV and 30% of group III afferents innervating incised muscle had spontaneous activity [28]. When examined using in vitro skin-nerve preparation, a greater proportion of C-fibers close to incision (≤ 2 mm to the incision) had spontaneous activity (40–48%), compared to C-fibers innervating non-incised plantar skin (7–13%) [29,30]. Similarly, a greater proportion of A-nociceptors close to incision showed ongoing activity (46%), compared to control A-nociceptors (5%) [30]. Spontaneous activity of nociceptors innervating incised tissue may account for non-evoked, spontaneous pain behaviors after incision.
2) Mechanical and heat sensitization: Using in vivo electrophysiological recording, we have demonstrated that primary afferents innervating the incised area are sensitized to mechanical stimuli [27,31,32]. When examined 45 min after plantar incision, both mechanosensitive Aδ- and C-fibers exhibited sensitization, evidenced by lower threshold, higher response magnitude, and greater receptive field size to mechanical stimuli compared to pre-incision baseline [31]. Furthermore, incision caused conversion of mechanically insensitive afferents to mechanosensitive fibers. Similarly, enhanced responsiveness of primary afferents to monofilament was apparent 1 day after plantar incision, compared to the sham surgery group [27,32]. Mechanical and heat sensitization of cutaneous and muscle nociceptors were also evident when examined using the in vitro muscle-nerve and in vitro skin-nerve preparations on postoperative day 1 [28,29,33]. Peripheral sensitization to mechanical and heat stimuli may have a role in maintenance of primary hyperalgesia after incision.
3) Chemical sensitization: While it long has been suggested that chemical mediators released around the site of tissue injury could lead to sensitization and activation of nociceptors, chemosensitivity of nociceptors in pathological states has been relatively understudied. We have undertaken a series of experiments to rigorously evaluate chemical sensitization of nociceptors after incision. First, we have evaluated acid-responsiveness of cutaneous nociceptors to lactic acid using the rat glabrous in vitro skin-tibial nerve preparation [30]. We used lactic acid as a low pH stimulus, based on our previous studies showing that pH is decreased and lactate concentration is increased in the incised tissue at the same time that pain behaviors are obvious [22,23]. The majority of chemosensitive nociceptors were C-fibers, and few A-fiber responded to lactic acid. When studied on postoperative day 1, C-fibers in the vicinity of the incision (≤ 2 mm to the incision) showed qualitatively and quantitatively greater responses to pH 6.0 lactic acid compared to control; a greater proportion (53%) of C-fibers from incision was chemosensitive compared to sham control (14%), and the median discharge rate during acid application was also greater (1.34 imp/s) in C-fibers from incision, compared to sham control (0.17 imp/s). Acid-responsive C-fibers from incised rats showed pH-dependent responses to lactic acid (pH 6.5 to 5.5; Fig. 3); greater acidity activated more C-fibers, and generated greater discharge rates during acid application. Enhanced responsiveness of C-fibers to lactic acid after plantar incision could be prevented by pre-treatment with dilute capsaicin, which desensitize C-fibers expressing transient receptor vanilloid-1 (TRPV1). This indicates that TRPV1-containgin fibers are major contributors to the chemical sensitization due to surgical injury [34].
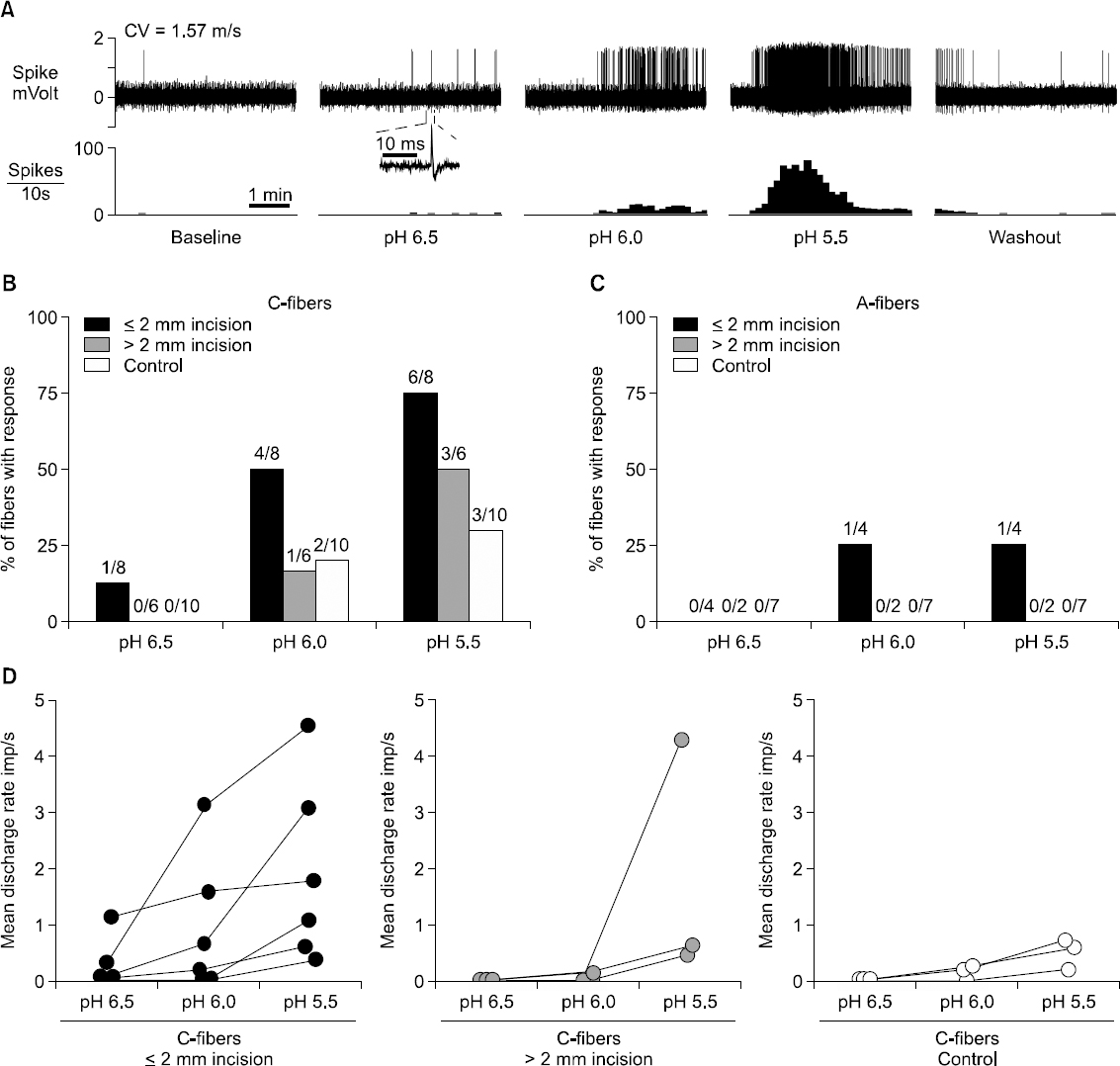
Summary of chemical responses of nociceptors to lactic acid of three different pH levels. (A) Sample recordings from one single C-fiber innervating ≤ 2 mm from the incision. After a 5-min baseline, 15 mM lactic acid with pH 6.5, 6.0 and 5.5 was sequentially applied for 5 min, followed by 5-min washout. The interval between each acid application was 15 min. The upper and lower panels show the digitized oscilloscope tracings and spike density histograms (bin width = 10 s), respectively. Inset displays the action potential of this unit. CV = conduction velocity. (B, C) Prevalence of acid-responsive units in C-fibers (B) and A-fibers (C). (D) Mean discharge rate of each acid-responsive C-fiber during application of 15 mM lactic acid with three different pH levels. Imp = impulse. Reprinted from Anesthesiolgoy, Vol. 111, S. Kang and T. J. Brennan, “Chemosensitivity and mechanosensitivity of nociceptors from incised rat hindpaw skin,” p 155-164, 2009, with permission from Wolters Kluwer Health, Inc.
Incision also increases chemosensitivity of muscle nociceptors. We evaluated responsiveness of muscle nociceptors to lactic acid using the in vitro plantar flexor digitorum brevis muscle-nerve preparation in rats [28,35]. One day after plantar incision, the proportion of chemosensitive units responsive to pH 6.0 lactic acid was greater in the incision group (65% of group III and 51% of group IV afferents) compare to un-incised control group (17% of group III and 22% of group IV afferents) [28]. Our previous study suggests that the decrease in pH in the incised muscle tissue was rather modest (∼ pH 6.8) [23]. Therefore, we have also examined response of the muscle nociceptors to weaker acid solutions, pH 7.0–6.5 [28,35]; one day after incision, the prevalence of chemosensitive group IV afferents to pH 6.5 lactic acid was greater in the incision group (22%), compared to that in the control group (6%). The optimal pH activating muscle nociceptors varied, some had greater responses to pH 7.0 than to pH 6.5. Together, our data support the possibility that an ischemic-like signal from the wound environment may lead to activation of sensitized nociceptors, contributing to postsurgical pain.
2. Pain mediators in the wound environment
One of the important mechanisms for peripheral sensitization is release of various pain mediators into the wound environment, leading to enhanced excitability and activation of nociceptors.
1) Ischemic-like pain mechanism: As briefly descried above, our previous studies using incisions in several tissues have demonstrated that tissue pH decreases and lactate levels increase for several days after incision [22,23]. Since protons and lactate are putative mediators of ischemic muscular pain, we hypothesized that wound environment would be hypoxic, creating an ischemic-like condition. To evaluate the time course of tissue hypoxia in the wound environment, we directly measured tissue oxygen tension using an oxygen-sensitive needle microelectrode in anesthetized rats, at several time points after incisions of the gastrocnemius muscle, the paraspinal skin, and the plantar hind paw [21]. Contralateral, un-incised tissues were used as control to minimize inter-subject variability. Tissue oxygen tension was significantly lower in the incised muscle during early postoperative period (11.3 ± 10.6 mmHg 1 h after incision and 5.55 ± 6.6 mmHg on postoperative day 1) compared to the control muscle (approximately 40 mmHg), and remained decreased through postoperative day 7 (Fig. 4). Tissue oxygen tension was decreased in the incised skin compared to control for several days after incision as well. We also evaluated in vivo hypoxia immunohistochemically, using a hypoxia marker pimonidazole hydrocholoride; consistent with the findings from direct measurement, pimonidazole immunostaining revealed hypoxic areas in incised muscle and skin for several days [21]. Taken together, a decrease in tissue oxygen tension occurs concurrently with a decrease in pH and an increase in lactate after incision (Fig. 4) [21-23], suggesting that these mediators in the wound environment may contribute postoperative pain through an ischemic-like mechanisms. Acid-sensing ion channel 3 (ASIC3) has been suggested to be a sensor for ischemic pain by detecting lactic acid [36,37], and pain behaviors after incision could be reduced by the ASIC3 antagonist, APETx2 [38], further supporting a significant role of ischemic-like mechanisms contributing to pain after surgery.

Time course changes in tissue pH, lactate concentration, and tissue oxygen tension after gastrocnemius muscle (A), paraspinal skin (B) and plantar (C) incision. A decrease in tissue oxygen tension occurs concurrently with a decrease in pH and an increase in lactate after incision. By postoperative day 10, tissue oxygen tension, lactate, and pH have returned to that of the control. The area shaded in gray represents the time after incision when pain-related behaviors are observed. Control is the value for the contralateral un-incised site. Error bars were omitted for simplicity. (Adapted and modified from reference [23] & [22]). Reprinted from Wound Repair Regen, Vol. 21, S. Kang and et al., “Wound hypoxia in deep tissue after incision in rats,” p 730-739, 2013, with permission from John Wiley and Sons.
2) Neurotrophic factors: Neurotrophic factors are regulators for survival and maintenance of neuronal cells during development and under physiological conditions. However, under pathological conditions like inflammation and nerve injury, neurotrophic factors are thought to play a role in sensitization of sensory neurons. Our previous studies have shown that pronociceptive neurotrophic factors, such as nerve growth factor (NGF) and artemin, were upregulated in injured cutaneous and muscle tissue after plantar incision in rats [18-20]. An increase of NGF expression in the wound environment occurred within hours after incision, and returned to baseline by postoperative day 7 [17-19]. Fibroblasts and Schwann cells adjacent to the injury seem to be sources of NGF in the incisional wound [18,19]. In line with these findings, sequestration of NGF via systemic pre-treatment with anti-NGF antibody or peptibody attenuated guarding behaviors in a dose-dependent manner, supporting the notion that NGF contributes to ongoing pain induced by incision [17,19]. Sequestration of NGF also attenuated heat hyperalgesia after incision, but did not influence responses to mechanical stimulation. These findings suggest that the mechanisms for exaggerated responses to heat stimuli and mechanical stimuli after incision may differ [17,39]. Overall, our results indicate that neurotrophic factors may contribute to acute pain after surgery.
3) Other mediators: Inflammatory cytokines in the wound environment may be involved in sensitization and activation of nociceptors, contributing to postoperative pain. Using quantitative, real-time reverse transcription polymerase chain reaction (RT-PCR), we have shown that pronociceptive cytokine interleukin-1 mRNA (IL-1) was upregulated in both skin and muscle after incision, and returned to near-baseline when pain behaviors resolved in rats [20]. In a study evaluating the role of IL-1 in postoperative pain, mice with genetic impairment of IL-1 signaling did not display mechanical hyperalgesia after plantar incision [40]. IL-1 receptor antagonist reversed mechanical hyperalgesia when given 24 h after incision, and prevented development of this pain behavior when given before surgery. Together, these findings suggest that IL-1 has a critical role in the development and maintenance of acute postoperative pain. We also have shown upregulation of IL-6 in incised skin and muscle [20]. Local treatment with resveratrol, which inhibits IL-6 release and IL-6-induced signaling, strongly attenuated mechanical hyperalgesia induced by either IL-6 injection or plantar incision in mice [41], suggesting a role of this cytokine in pain-related behavior after surgery.
Complements produced in wounds may contribute to postoperative pain [16]; C5 protein levels were increased in skin wounds as early as 2 h and remained high at least 72 h after plantar incision in rats. mRNA levels of C5 and C5a receptor in the skin were also increased after incision. Local treatment with the C5a receptor antagonist via intraplantar injection attenuated the heat and mechanical hyperalgesia induced by either C5a injection or plantar incision. There results indicate that high local concentrations of C5a produced in the wound area likely contribute pain-related behavior after surgery.
3. TRPV1-containing nociceptors
TRPV1 has been considered as a molecular signal integrator in nociceptors. For incisional pain, the TRPV1 antagonist AMG0347 did not affect guarding pain behavior or mechanical hyperalgesia, and only decreased heat hyperalgesia in rats [42]. Similarly, while knockout of TRPV1 abolished heat hyperalgesia after plantar incision, no differences were observed in the guarding behaviors and mechanical hyperalgesia between TRPV1 knockout and wild-type mice [43]. On the other hand, treatment with dilute capsaicin (0.025–0.10%), either via perineural application or local infiltration, significantly reduced guarding pain and heat hyperalgesia after plantar incision; there was minimal effects on mechanical responses [34,44]. To understand the mechanisms for the selective action of dilute capsaicin on incisional pain, we examined the effect of capsaicin treatment on the activity of nociceptors using the rat in vitro glabrous skin-nerve preparation [34]; the same dose of capsaicin that inhibited guarding behavior and heat hyperalgesia interfered with responsiveness of C-fibers to heat and chemical stimuli, pH 5.5 lactic acid, while mechanosensitivity of nociceptors was unaffected. Capsaicin treatment also decreased calcitonin gene related peptide and isolectin B4 (IB4)/protein gene product 9.5 (PGP9.5)-immunoreactivity of nociceptors. These results indicate that nociceptors desensitized by capsaicin contribute to guarding pain and heat hyperalgesia after plantar incision. Together, our previous reports suggest that TRPV1-expressing afferent fibers, but not TRPV1 receptor itself, are critical for incision-induced guarding pain; other receptors on TRPV1-expressing fibers may have an important role. Our results also imply that various pain-related behaviors in plantar incision model are mediated by separate mechanisms.
Central sensitization
Central sensitization is characterized by increased responsiveness of nociceptive neurons in the central nervous system. Secondary hyperalgesia is believed to be mainly due to central sensitization [45,46], and perhaps it may be related with increased risk of chronic post-surgical pain.
1. Dorsal horn neuron activity after incision
To better understand the changes in spinal sensory processing caused by surgery and its contribution to postoperative pain, we have undertaken a series of neurophysiological experiments to evaluate activity of dorsal horn neurons in the rat plantar incision model. When recorded from lumbar dorsal horn neurons whose receptive fields included at least part of the injury, plantar incision resulted in sensitization of these neurons, which manifested in markedly increased ongoing spontaneous activities, expansion of receptive fields, and enhanced responses to mechanical stimuli [24-26,47]. Injection of local anesthetic into the incision site reversed the spontaneous activity of dorsal horn neurons to the level of sham-operated rats, indicating that increased spinal neuron activity after surgery is induced and maintained largely by primary afferent input from the incision [24-26].
It appears that separate groups of dorsal horn neurons transmit spontaneous activity and enhanced responses to mechanical stimuli after plantar incision [24]; in a subset of dorsal horn neurons, those without spontaneous activity, the responses to mechanical stimuli were much greater the incision group compare to the control group, suggesting that these neurons could transmit the mechanical hyperalgesia after surgery. On the other hand, the same mechanical stimuli elicited only weak response in another subset of dorsal horn neurons, those with high ongoing, spontaneous activity, suggesting that these neurons may signal ongoing pain after surgery.
2. Spinal non-N-methyl-D-aspartate (non-NMDA) excitatory amino acid receptors
Experimental evidence by others indicates that spinal N-methyl-D-aspartate (NMDA) receptors or spinal metabotropic glutamate receptors (mGluR) have a role in pre-clinical models of persistent pain. However, intrathecal administration of NMDA receptor antagonists or mGluR antagonists did not modify pain-related behaviors after plantar incision in rats, indicating that these receptors do not play an important role in postoperative pain models [48-50]. On the other hand, intrathecal or epidural administration of non-NMDA excitatory amino acid (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid [AMPA] or kainate) receptor antagonists significantly attenuated guarding behaviors and mechanical hyperalgesia induced by plantar incision [13,49,51]. Thus, non-NMDA receptors seem to play an important role in maintaining acute postoperative pain behaviors. Consistent with these findings, the non-NMDA receptor antagonist NBQX, but not NMDA antagonist MK-801, inhibited sensitization of dorsal horn neurons after plantar incision [52]; NBQX, but not MK-801, effectively inhibited incision-induced spontaneous activity, mechanical hypersensitivity, and expansion of receptive fields in spinal dorsal horn neurons. Furthermore, incision produced a different pattern of excitatory amino acid release in the lumbar dorsal horn, compared to other persistent pain models [53]; overall, the percentage increase was less and for a shorter period of time in plantar incision model. Plantar incision induced a short-lived increase in glutamate and aspartate release, lasting only for 45 min, and the concentrations of these amino acids returned to baseline by 1 h after incision. Together, these findings indicate that the pathophysiological mechanisms associated with incision may be different from those underlying inflammatory, neuropathic, or chemical irritant-induced pain models.
We have examined a role of spinal NMDA and non-NMDA (AMPA/kainate) receptors for secondary hyperalgesia caused by gastrocnemius incision in rats [46]; secondary mechanical hyperalgesia after gastrocnemius incision was dose-dependently blocked by spinal non-NMDA receptor antagonists, but only marginally affected by competitive or non-competitive NMDA antagonists. To further clarify witch receptor subtypes are critical for incision-induced secondary hyperalgesia, we then evaluated a role of spinal Ca2+ permeable non-NMDA receptors in this model [46]. Secondary mechanical hyperalgesia was reversed by Joro spider toxin, an antagonist for Ca2+ permeable AMPA/KA receptors, indicating that spinal sensitization contributing to secondary hyperalgesia after incision requires Ca2+ permeable AMPA/KA receptors. On the other hand, guarding behaviors and primary mechanical hyperalgesia were unchanged by Joro spider toxin. There data indicate that mechanisms for central sensitization causing secondary hyperalgesia in postoperative pain models may be separated from the peripherally driven mechanisms for spontaneous pain or primary mechanical hyperalgesia.
3. Preventive analgesia vs. pre-emptive analgesia
The concept of pre-emptive analgesia was based on the hypothesis that treatment that starts before surgical incision would prevent the establishment of central sensitization and reduce the subsequent pain experience, with an assumption that central sensitization has a major contribution to postoperative pain. In several pre-clinical models of persistent inflammatory or neuropathic pain, it has been shown that pre-emptive treatment initiated before tissue injury to block nociceptive input to the spinal cord reduced subsequent pain-related behaviors. However, pre-emptive analgesic effect was less evident in our previous studies using the models of postoperative pain; early reduction in pain-related behaviors by pre-incision treatments with spinal morphine, bupivacaine, or non-NMDA receptor antagonists had no impact on subsequent pain behaviors beyond the expected duration of the expected analgesic effect [49,54]. These data suggest that incisional pain may be initiated and maintained differently than persistent pain models. As stated earlier, peripheral sensitization makes a major contribution to the development and maintenance of post-incisional pain-related behaviors [24-26]; when the analgesic effect of pre-incision treatments diminishes, it is likely that the surgical wound is capable of generating same mechanisms maintaining postoperative pain, producing pain behaviors equivalent to the untreated group. These findings from our pre-clinical models may explain why many clinical studies have failed to show any meaningful benefit of pre-emptive analgesia, compared to analgesics administered after surgery. Timing of initiation of analgesia may not be as important as the duration and efficacy of analgesic regimens. Therefore, efforts to minimize neuronal sensitization and hypersensitivity should be extended into the postoperative period (“preventive analgesia”).
CONTRIBUTION OF DEEP MUSCLE TISSUE TO ONGOING PAIN AND SPONTANEOUS ACTIVITY OF NOCICEPTIVE PATHWAYS
Guarding behavior after plantar incision may be translatable to resting pain in patients after surgery. Similar to the time course of resting pain in patients after surgery, guarding behavior is the greatest immediately after incision, and gradually resolves over several days [10]. However, when incision was made through the receptive field of the primary afferents or dorsal horn neurons in anesthetized rats, spontaneous activity was not evident immediately after plantar incision [31,47]. Spontaneous activity of primary afferents and dorsal horn neurons was much greater, however, when examined on postoperative day 1 [24,25,27]. The reasons for this discrepancy between behavioral and neurophysiological data are unclear; perhaps when the immediate effects of incision were examined, there was a greater tendency to record from nociceptors and dorsal horn neurons that had predominately cutaneous input. We hypothesized that strong guarding pain and ongoing activity of nociceptive pathways would be produced by skin plus deep tissue (fascia and muscle) incision, while skin-only incision would have minimal contribution to the development of guarding pain and spontaneous activity. To test this hypothesis, we have conducted a series of experiments examining the behavioral and neurophysiological effects of skin plus deep tissue plantar incision, compared to skin-only incision or a sham procedure in rats [26,32]. First, we evaluated incision-induced pain behaviors in these three groups of animals [32]; compared with the sham procedure, skin-only incision produced moderate guarding (cumulative pain score 5.9 ± 2.2) on the day of incision only, and did not produced guarding behavior on postoperative day 1 and thereafter. Skin plus deep tissue incision caused greater and longer guarding pain from 2 h (19.1 ± 1.1) to postoperative day 5 (5.1 ± 1.1). These data indicate that incised deep tissue, rather than skin, has a critical role in the genesis of guarding behavior. In contrast, skin incision alone was sufficient to induce hypersensitivity to mechanical and heat stimuli.
Next, we performed in vivo single-fiber recording after a sham procedure, skin-only, or skin plus deep tissue incision [32]. Overall, spontaneous activity of nociceptors was strongly associated with guarding behavior after plantar incision. On postoperative day 1, the prevalence of spontaneous activity in nociceptors was greater when the deep tissue was incised (61%), compared to that of the sham control (13%) or skin-only incision group (18%). Changes in spontaneous activity in nociceptors correlated with the time course of guarding behavior; a marked increase in spontaneous activity occurred when guarding behavior was apparent on postoperative day 1; the prevalence of spontaneously active nociceptors on postoperative day 7 was similar to the sham control group, as guarding behavior resolved.
Recordings from the dorsal horn neurons were in agreement with the findings from the primary afferent nociceptors, further supporting a strong relation between guarding pain and spontaneous activity in nociceptive pathways [26]. On postoperative day 1, the prevalence of spontaneously active dorsal horn neurons was greater after skin plus deep tissue incision (78%), compared to that of the sham control group (36%). The skin-only incision group had a similar percentage of spontaneously active neurons (53%) as the sham group. The rates of spontaneous activity also tended to be greater after skin plus deep tissue incision, compared to the control group. Infiltration of bupivacaine into the incision decreased spontaneous activity to the same level as the sham-operated rats, suggesting that robust spontaneous activity of dorsal horn neurons after incision is largely maintained via peripheral input from the incision. Seven days after skin plus deep tissue incision, animals did not exhibit guarding behavior, and the percentage and amount of spontaneous activity of dorsal horn neurons from these animals were the same as the sham control. Taken together, our results indicate that deep muscle tissue rather than skin is critical for the development of ongoing pain and increased spontaneous activity of nociceptive pathways, whereas incised skin is sufficient to induce mechanical and heat hyperalgesia.
The important role of deep tissue injury in genesis of postoperative pain has been demonstrated in human postoperative pain as well [55,56]. In a prospective, randomized, blinded study, two different surgical approaches for a unilateral total hip arthroplasty were compared: (1) a conventional arthroplasty and (2) a minimally invasive approach with a lesser extent of muscle injury [56]. Both approaches used the same length of skin incision. Compared with conventional approach, minimally invasive approach resulted in significantly less postoperative pain. On the other hand, in patients undergoing a unilateral hip arthroplasty, approaches with different lengths of skin incision did not produce any difference in postoperative pain when the same degree of deep tissue injury occurred [55].
CLINICAL IMPLICATIONS OF POSTOPERATIVE PAIN MODELS
Data from human studies support the findings from the incisional pain models, reinforcing the translational potential of these pre-clinical models.
Human model for postoperative pain
Pain induced by surgical skin incision has been characterized in human volunteers [45,57]; an experimental 4-mm-long incision was made in the volar forearm, and pain assessment was performed during and after incision. Spontaneous pain, rated using the visual analog scale, was maximal when the incision was made (40–50 mm) and rapidly disappeared within 30 min to 1 h after the incision; no sustained spontaneous pain was present. Primary hyperalgesia to punctate mechanical stimuli was apparent as early as 15 min after the incision and maintained for longer period of time, lasting for 2–3 days. Secondary mechanical hyperalgesia was apparent at 30 min after incision, and then gradually disappeared over the next 3–6 h. Overall, these data suggest that incision of skin causes significant primary mechanical hyperalgesia, and has only a transient effect on spontaneous pain and secondary hyperalgesia. Lidocaine, locally injected 10 min before incision, delayed development of primary hyperalgesia only for up to 4 h; afterwards, the mechanical responsiveness of lidocaine-treated group was not different from that of vehicle-treated group. While lidocaine given before incision prevented development of secondary hyperalgesia, fully developed secondary hyperalgesia was not modulated by post-incisional injection of lidocaine, indicating an important role of central sensitization in the maintenance of secondary hyperalgesia.
Postoperative pain in patients
Analgesic drugs with known clinical efficacy in postoperative pain management in patients, such as opioids, NSAIDs, and local anesthetics, have been shown to be effective in modifying pain behaviors in the plantar incision model, supporting the validity and reliability of this pre-clinical pain model [10,12,20,54]. Capsaicin is a good example as well. As stated above, perineural application or local infiltration of dilute capsaicin (0.025–0.10%), a selective agonist of TRPV1, significantly reduced guarding behaviors induced by plantar incision in rats [34,44]. In agreement with these pre-clinical studies, clinical studies suggest analgesic efficacy of capsaicin in postoperative pain. In a prospective, randomized, double-blinded study, single intraoperative wound instillation of dilute capsaicin (0.006%) resulted in superior analgesia compared to placebo during the first 3–4 days after inguinal hernia repair [58]. In another randomized, double-blinded study, wound instillation of capsaicin prior to wound closure resulted in significant opioid sparing and lower pain scores during the first 3 postoperative days after total knee arthroplasty, compared to the placebo group [59]. Together, these data indicate that capsaicin, locally administered into surgical wounds, is a candidate for novel, long-lasting analgesic for moderate to severe pain after surgery. Another example that further supports translatability of plantar incision model would be gabapentinoids. Gabapentinoids, such as gabapentin and pregabalin, have anti-hyperalgeisic and anti-allodynic properties, and have been widely used for management of neuropathic pain. Gabapentinoids was shown to be effective in attenuating incision-induced mechanical hyperalgesia in pre-clinical studies using the plantar incision model [60], suggesting its potential role in the management of postoperative pain. Systemic reviews and meta-analyses of clinical trials have shown that gabapentinoids reduced pain and opioid consumption in patients during early postoperative period [61,62]. Although more studies are warranted to determine the optimal dosing regimens and the long-term benefits, the evidence so far suggests the surgical patients may benefit from perioperative gabapentinoids.
CONCLUSION
Evidence suggests that the pathophysiological mechanisms and optimal treatment of postoperative pain are different from many other painful conditions. Recognizing the necessity and importance of relevant pre-clinical models, we have developed and characterized rodent incisional models that have close similarities to postoperative pain in patients. Previous studies have demonstrated the clinical relevance and translatability of these pre-clinical models of postoperative pain. These models have substantially improved our understanding of pain caused by surgery, and we continue to advance our knowledge on the pathophysiology of incisional pain. A better understanding of the mechanisms of postoperative pain will lead to the development of novel treatment strategies that will greatly reduce postoperative pain.