Unexpected anesthetic leakage from a damaged O-ring on the Selectatec back bar -A case report-
Article information
Abstract
The Selectatec mounting system was devised to provide easy and quick on-site fitting of various vaporizers for the anesthetic machine. However, a quick changing system for the vaporizer can also damage the O-ring due to friction between the vaporizer and the Selectatec back bar. We herein report a case of an unexpected anesthetic gas leakage from a damaged O-ring on the Selectatec back bar, which resulted from exchanging the vaporizers between two operations. In cases using the Datex Ohmeda machine, it is not easy to detect leakages from the vaporizers because of the location of the check valve near to the fresh gas outlet. This complicates the use of the positive pressure leakage test to detect a low pressure system leakage on the Selectatec back bar. We recommend the preanesthetic negative pressure or low-flow leakage test to detect a low pressure leakage when exchanging vaporizers on the Selectatec system.
INTRODUCTION
Anesthesia machines need to be handled with precision because their malfunction is one of several causes of anesthetic complications, including intraoperative awareness [1]. Prior to a single anesthetic episode, anesthesia machines should be leak tested. However, such leakage tests should be tailored to the specific device as anesthesia machines differ in their configuration. Here, we report a case of sevoflurane leakage with anesthetic gas through a damaged O-ring in the Selectatec back bar of the anesthesia machine, which was not detected antecedently because of incomplete testing.
CASE REPORT
A 50-year-old female patient (weight = 70.6 kg; height = 162 cm; body mass index = 27.0) was scheduled to undergo a left modified radical mastectomy for breast angiosarcoma at our hospital. This patient was intraoperatively monitored using pulse oximetry, noninvasive blood pressure, and electrocardiography. We also placed a Bispectral index (BIS) monitor (Aspect Medical Systems, USA) on the left temporal- frontal area of the forehead. We used the electronic system check of the anesthesia machine we were using, the Ohmeda Aestiva/5 machine anesthesia workstation (Datex-Ohmeda, GE Healthcare, UK), and confirmed that this instrument, its vaporizer, and other components such as tubing, flow meters, valves, gaskets, scavenging system, etc. were working normally. The circle breathing circuit was also manually checked using common gas outlet occlusion test before anesthetic induction.
The patient underwent preoxygenation with 100% O2 and then 2 mg midazolam, 120 mg propofol, and 14 mg cisatracurium were intravenously administered to induce anesthesia. Manual ventilation with 3 vol% sevoflurane and 8 L/min oxygen under the continuous infusion of remifentanil was performed using the Ohmeda Aestiva/5 machine with a Tec 7 vaporizer. When inducing anesthesia, we detected a leaking sound from the vaporizer and confirmed its position. We increased the inhaled concentration of sevoflurane because the BIS had increased to > 60. However, even though the dial concentration was set to 3 vol% sevoflurane, the gas analyzer detected less inhaled sevoflurane and the end-tidal anesthetic gas concentration of the volatile agent showed 0.6%. At the same time, the patient’s BIS value was > 70 (Fig. 1).

Time course of bispectral index (BIS) and end-tidal anesthetic agent (EtAA) monitoring. Bold dot lines, BIS values. Thin red lines, EtAA concentrations. Phase 1: unexpected sevoflurane leakage. Phase 2: manual bagging, during which the medical engineering team found the damaged O-ring. Phase 3: after O-ring replacement, the anesthesia machine worked well.
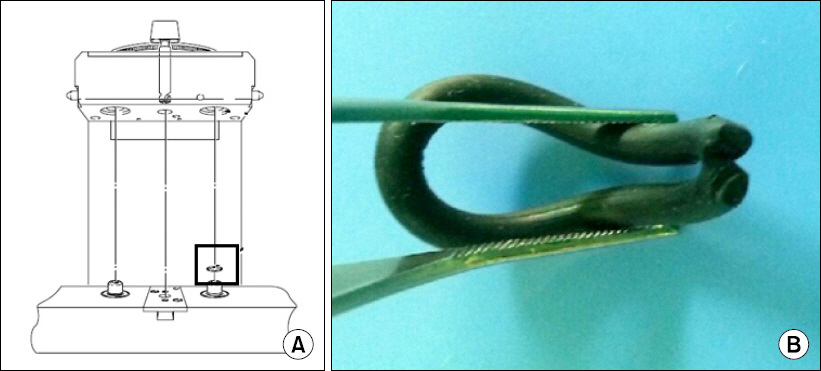
Both 3 mg midazolam and 60 mg propofol were intravenously injected to prevent patient awareness. To check the vaporizer functions, we disengaged its locking lever and carefully lifted it from the Selectatec back bar. However, we could not find any problems and we thus reinstalled the vaporizer into the manifold. We turned the dial after we ensured that it had been correctly mounted but it failed to work again. We doubted that the vaporizer was defective because the inhaled concentrations of the anesthetic agents did not increase. We next turned off the vaporizer and set the gas flow to 100% O2. We then immediately called the medical engineering team and manual ventilation was continued. Three mg midazolam and 60 mg propofol were intravenously administered again to prevent patient awareness. Within 5 minutes of inspecting the device, the medical engineering team located a damaged O-ring in the manifold port valve of the Selectatec back bar (Fig. 2). Meanwhile, the patient’s vital parameters were carefully checked to ensure that they remained stable. After replacing the O-ring, there was no further leakage. Intraoperative anesthesia was subsequently maintained with sevoflurane 2 vol%, medical air 2 L/min, and O2 1 L/min using this semiclosed-circuit anesthesia machine. The intraoperative vital signs of the patient were stable and the surgery was safely completed. No patient awareness during general anesthesia was observed and she was eventually discharged without any complications.
DISCUSSION
Although anesthesia machines undergo routine diagnostic maintenance it is not easy for anesthesiologists to determine all leakage sites because each part of these instruments is composed of complex components that are not airtight [2]. Leaks within the anesthetic machine and breathing system make up 9.6% of incidents during anesthesia, of which 5.1% are related to the vaporizer and 1.9% to the supply of gas to the machine. The most common locations of a leak are the low pressure system, breathing system, or around endotracheal tube [3].
Anesthesia machines are connected to low- and high-pressure systems. The lines that deliver oxygen, nitrous oxide, and medical air to these devices are part of the high-pressure system, and leakage can occur at disconnected high-pressure lines. To prevent high-pressure leakage, anesthesiologists need to check the oxygen cylinder and central pipeline [4]. The low-pressure system in these instruments includes the vaporizer, flow meter, common gas outlet, and patient breathing circuits after lowering the gas pressure via a regulator. Because the components located within this area are the most prone to breaks and leaks, low-pressure leakage develops more frequently than high-pressure leakage [5].
The Selectatec mounting system provides a simple “hang on/lift off” mounting system for various vaporizers, including halothane, enflurane, isoflurane, sevoflurane, and desflurane. However, at the same time, this quick changing system can damage the O-ring, thereby risking hypoventilation, rebreathing, oxygen dilution, and awareness due to friction between the vaporizer and Selectatec back bar [6,7]. Patient awareness that resulted from faulty internal seals in the Selectatec manifolds has been previously reported [8].
Several different methods can be used to detect low-pressure leakage, such as the common gas outlet occlusion test, oxygen flush test, and positive-pressure leak test [9]. However, the Datex-Ohmeda workstations complicate the use of the common positive-pressure leak test to assess low-pressure systems because the outlet check valve is located upstream from the machine’s fresh gas outlet in these systems [9]. The manufacturer GE Healthcare thus recommends performing the negative-pressure leak test with a special suction bulb device or universal negative-pressure leak test to locate any leakage from the low-pressure system [9,10].
Notably, many of the newer generation anesthesia machines do not have an accessible common gas outlet and manual low pressure system testing cannot therefore be performed. These machines would most likely test the integrity of the low pressure system via an automated checkout. However, some machines of the current generation still require a manual low pressure system leak test, such as the Datex-Ohmeda Aestiva/5®, Datex-Ohmeda Aisys® with ACGO, Datex-Ohmeda S/5 Aespire®, Narkomed 2B®, Narkomed M®, Narkomed MRI®, and Penion Prima SP3® systems. For those that do, the universal leak test can be conducted unless clearly instructed otherwise by the manufacturer [11]. However, this method is somewhat time- consuming and involves an additional test to inspect the patient’s breathing circuit because it can detect leakage only in the low pressure system.
Unlike the negative-pressure leak test, the low-flow leak test (LFLT) recommended by the Japanese Society of Anesthesiologists can test the entire anesthetic circuit and be performed on anesthesia machines with a check valve [12]. The LFLT is carried out under the following conditions: (1) the tube end of the Y-piece is connected to the breathing bag; (2) the oxygen flow is set at 0.1 L/min (or at the minimal basal flow); (3) the manometer on the machine is carefully monitored to ensure that the pressure in the breathing circuit has reached 30 cmH2O; and (4) when the pressure reaches 30 cmH2O, then no leak could be larger than 0.1 L/min. A major advantage of this test is very time-saving and its reliability for leak detection [12].
In our current case, we assumed that O-ring damage might have developed after replacing the vaporizer from desflurane to sevoflurane prior to surgery. We could have found this leak if we had conducted the negative-pressure or low-flow leak test, but we instead performed a common gas outlet test. Fortunately, by using real-time BIS monitoring and an anesthetic gas analyzer, we rapidly detected the leak and tool several prophylactic measures to prevent patient awareness due to the malfunctioning of the anesthesia machine. Hence, if the anesthesiologist wants to change the vaporizer in the Selectatec system, it is recommended to verify that the O-ring in the manifold port valve is intact. In addition, the anesthesiologist should be well acquainted with the concepts that underlie the subsystems of modern anesthetics and should not disregard any defects in these systems. Although advances in patient- monitoring skills and the automated check functions of the latest anesthesia machines help to prevent many problems, it is still necessary to be well-informed on the appropriate pre-use inspections of anesthesia equipment [13,14]. Therefore, prior to initiating anesthesia, it is required to perform pre-use leak tests using the checklists for anesthesia equipment issued in 1993 by the United States Food and Drug Administration [15].