INTRODUCTION
It is exceedingly difficult to detect the pain occurring during surgery under general anesthesia accurately and to perform appropriate pain control, and the level of analgesia provided during the operation is fundamental, because it is directly linked to postoperative pain and complications. This is especially important for pediatric patients, because it is more challenging to communicate with them than adults during the perioperative period. Moreover, postoperative emergence and recovery patterns of pediatric patients are different from those of adults, and it is also difficult to predict the degree of postoperative pain (due to a limitation of pain rating scales). Therefore, if the degree of nociception (pain) during surgery is objectively predicted and evaluated, thereby appropriately controlled in such pediatric patients, postoperative pain can be effectively reduced, and it may be helpful to perform additional analgesia for residual pain and to prevent postoperative complications.
In addition, while regional anesthesia (analgesia) can be performed in an arousal state in adults and the evaluation of its effectiveness (success or failure) is possible, it is exceedingly difficult to perform the procedure in an arousal state in pediatric patients. In this case, the evaluation of the success of regional analgesia becomes more difficult because the procedure is performed during general anesthesia. If there is a nociception monitoring device that can appropriately evaluate the effect of local anesthetics administered for regional analgesia, by monitoring before and after regional analgesia, it is possible to evaluate the effectiveness of regional analgesia and to judge its success. In this respect, intraoperative nociception monitoring can be beneficial for perioperative pain management in pediatric patients.
During the last two decades, several nociception monitoring tools have been developed, which utilize physiological markers that are useful for performing objective pain assessments. Of these, the most common method is to measure the degree of pain by measuring changes in the autonomic nervous system (ANS) activity [1]. Depending on which ANS surrogate marker—including pulse wave amplitude and pulse beat interval, heart rate variability, skin sweating, and pupillary changes—is used by each device, the basic operating principles and characteristics of the device (surgical pleth index, analgesia nociception index, heart rate variability, skin conductance, or pupillometry etc.) are determined. This article reviews the efficacy, reliability, and limitations of the devices for intraoperative analgesia in pediatric patients undergoing general anesthesia by analyzing the results of the studies on the nociception monitoring devices for pediatric patients reported so far—even though the results are fewer than those for adults [2]. A better understanding of the nature of these devices may help provide more effective and safe perioperative analgesia to pediatric patients undergoing surgeries using general anesthesia.
LITERATURE SEARCH STRATEGIES AND RESULTS
A literature search for the review was performed on major international and South Korean databases (PubMed, Embase, Cochrane, Web of Science, Scopus, and KoreaMed) to identify articles including systematic reviews, meta-analyses, practice guidelines, narrative reviews and clinical trials published since 1990 that assessed nociception or pain using nociception monitoring tools or indices for analgesic guidance in pediatric patients undergoing surgery using anesthesia. Databases were searched with the strings made using the Medical Subject Headings (MeSH) and free text terms (Analgesia; Anesthesia, general; Autonomic nervous system; Children; Monitoring, intraoperative; Nociception test; Pain measurement etc.). After the initial electronic search, the author evaluated the bibliographies of the identified studies and performed a manual search using Google Scholar. The articles identified were assessed individually for inclusion in the analysis.
Searches of the databases yielded 1,010 articles (Fig. 1). Of these, 950 publications were excluded, because it was clear from the title and abstract that they did not fulfill the selection criteria. From the remaining 60 articles, 30 potentially relevant studies were identified by scrutinizing the full-text articles. Thirty other publications were excluded because they included adult patients, or did not include available interventions (pain measurement with nociception monitoring tools or indices using autonomic tone changes in pediatric patients undergoing surgery under anesthesia). Therefore, 30 studies were finally included in this review (Fig. 1).
ANS MARKERS FOR NOCICEPTION ASSESSMENT
Postoperative pain increases ANS activity (i.e., increases sympathetic nervous system activity and postoperative analgesia suppresses the response) [3-5]. Similarly, nociception caused by surgical stimulation during general anesthesia causes the release of stress hormones, and the level of stress hormone release depends on the level of analgesia provided [6,7]. This is because there is a neuroanatomic overlap between the pain transmission path and the ANS pathway [8]; these rationale and findings have led to the assumption that pain causes a change in ANS activity, which has resulted in the development of several monitoring devices using ANS surrogate markers for objective pain assessment. Derived cardiovascular and respiratory parameters including pulse wave amplitude, pulse beat interval, heart rate variability, the pattern of blood pressure, and heart rate responses are representative ANS surrogate markers [1]. In addition, sweating and pupillary changes are also used as ANS markers for nociception monitoring [1].
Although studies on various nociception monitoring devices have been conducted on adults, recent research results on pediatric patients have also been reported. It is essential to understand the efficacy and limitations of the nociception monitoring tools in pediatric patients undergoing general anesthesia by analyzing existing literature and research results, especially to recognize the differences between the pediatric and adult results, in order to appropriately provide intraoperative analgesia based on these monitoring devices. Therefore, in addition to the 30 pediatric studies included in this study, the representative studies performed by applying the nociception monitoring tools used in the pediatric studies to adults were also cited and compared in this review.
NOCICEPTION MONITORING TOOLS USING PULSE WAVE AMPLITUDE AND PULSE BEAT INTERVAL
Surgical pleth index (SPI)
SPI, also called the Surgical Plethysmographic Index or Surgical Stress Index, is a simple, non-invasive monitoring tool used to assess nociception during anesthesia by analyzing the waveform and heartbeat of photoplethysmography in pulse oximeters. SPI is calculated using the pulse photoplethysmographic amplitude (PPGA) and heartbeat interval (HBI), which are measurable in the photoplethysmography [9] as follows in this equation,
SPI values range from 0 (no stress) to 100 (maximum stress level), and in previous studies, an SPI range of 20-50 usually indicates adequate analgesia during surgery under general anesthesia [10-13]. It is also recommended to keep SPI values below 50 and avoid rapid increases (more than 10-point increase over a short time) as another criterion to maintain adequate analgesia [14].
The effectiveness of SPI as a tool for monitoring and evaluating nociception and administering analgesics in adults was demonstrated in several studies [10-13,15]. Notably, they have shown that SPI-guided analgesia offers several clinical benefits in comparison to analgesia based on conventional hemodynamic parameters during surgery under general anesthesia [10,12,13,16,17]. In contrast, limitations have been reported for various clinical situations and confounding factors [18-23], one of which may be age. Thus, limitations presented in the studies of pediatric patients must be considered carefully [19,24,25].
Although the number of studies related to SPI in children is limited, efforts to reduce the incidence of postoperative pain and complications by appropriately evaluating nociception during surgery and performing appropriate analgesia have been attempted in various forms. The results have been reported in several ways, also [19,24-26]. Harju et al. observed changes in intraoperative SPI in two groups receiving ultrasound-guided ilioinguinal and iliohypogastric nerve block with saline or ropivacaine before the beginning of surgery after anesthesia induction in patients under 24 months old undergoing inguinal hernia surgery. As a result, an increase in SPI was observed in both groups after endotracheal intubation, whereas an increase in SPI during surgical incision was observed only in the saline-injected group. Harju et al. [26] reported that the reactivity of SPI to surgical stimuli was blunted in the ropivacaine-injected group, suggesting that SPI monitoring may be useful for determining the analgesic effect of the nerve block in pediatric patients undergoing regional analgesia, but also reported that the reactivity of the SPI was rather small, and there was a limitation of marked inter-individual variability in SPI reactions to nociception. More importantly, several other pediatric studies about SPI have shown that children have less confidence in SPI than adults, and thus children should receive intraoperative analgesia based on SPI levels that differ from those of adults. Supporting this, Park et al. [19] found that the cardiovascular structure and function of children—especially the vascular distensibility—is different from adults and that the actual nociception levels in children are not reflected in SPI values and the pediatric SPI value tends to be lower during surgery. A possible explanation is that ultrasonographic examinations of vascular structures showed lower vascular wall stress and higher distensibility in children than in adolescents [27], and basal catecholamine concentrations and resting muscle sympathetic nerve activity are also lower in children than in adults [28]. Due to these characteristics, the stimulation of vascular smooth muscle and vascular contractile force that occur during the activation of the sympathetic nervous system may be less in children. Two factors determine SPI values, HBI and PPGA, of which PPGA depends on the vascular wall distensibility and intravascular pulse pressure [29] and has double the effect on SPI values compared to HBI (Equation 1). Because children have lower vascular contractility and higher vascular distensibility than adults, activation of the sympathetic nervous system due to nociception is unlikely to significantly reduce PPGA as in adults, which can result in underestimation of SPI values.
The notion that there is less reduction in PPGA in children than in adults due to the unique characteristics of children’s vascular response at the time of nociception is supported by comparing the SPI data of previous studies. Compared with the results of two studies that reported changes in SPI, heart rate (HR), and PPGA during endotracheal intubation after administration of 2 μg/kg of fentanyl for anesthesia induction [30,31], the change in SPI values (the amount of increase) after endotracheal intubation was relatively higher in the adult study [30], while the change in HR was similar. Therefore, it can be inferred that the amount of change in PPGA, that is, the amount of decrease in PPGA, would have been higher in adults. Indeed, the median difference for PPGA before and after intubation reported in the pediatric study [31] was small (-0.51). Fig. 2 schematically shows the data from these two studies and the changes which occur in the photoplethysmography in adults and children at the time of nociception, the difference between them, and how the SPI values are changed.
Fig. 2
Comparison of photoplethysmographic (PPG) responses between adults and children for nociception during anesthesia. The schematic diagram shows the difference between adults and children on the change of the PPG signal during endotracheal intubation at anesthesia induction. The changes (decrease) of the photoplethysmographic amplitude (PPGA) and area under the curve (AUC) are smaller in children, and the resulting changes (increase) of the surgical pleth index (SPI) values are also smaller in children (refer [SPI ∞ 1/AUC]). That is, this difference in PPG responses for nociception may cause a difference in SPI values between adults and children. (A) PPG waveforms in adults. With an assumption that heartbeat interval (HBI) equals to PPGA (HBI = PPGA = α) and the data of SPI and heart rate in the reference (Mustola et al. Anesthesiol Res Pract 2010; 2010: 810721 [30]), the following equations can be established and thus the values of the variables can be assumed: α’ = α - 6.2 = 55.8 - 6.2 = 49.6; β = α - 22.8 = 55.8 - 22.8 = 33.0. (B) PPG waveforms in children. With an assumption that HBI equals to PPGA (HBI = PPGA = γ) and the data of SPI and heart rate in the reference (Kallio et al. Br J Anaesth 2008; 101: 383-9 [31]), the following equations can be established and thus the values of the variables can be assumed: γ’= γ - 8.2 = 60.8 - 8.2 = 52.6; δ = γ - 17.5 = 60.8 - 17.5 = 43.3. This figure is newly drawn based on the data of the two studies [30,31] that reported changes in SPI, heart rate, and PPGA during endotracheal intubation.
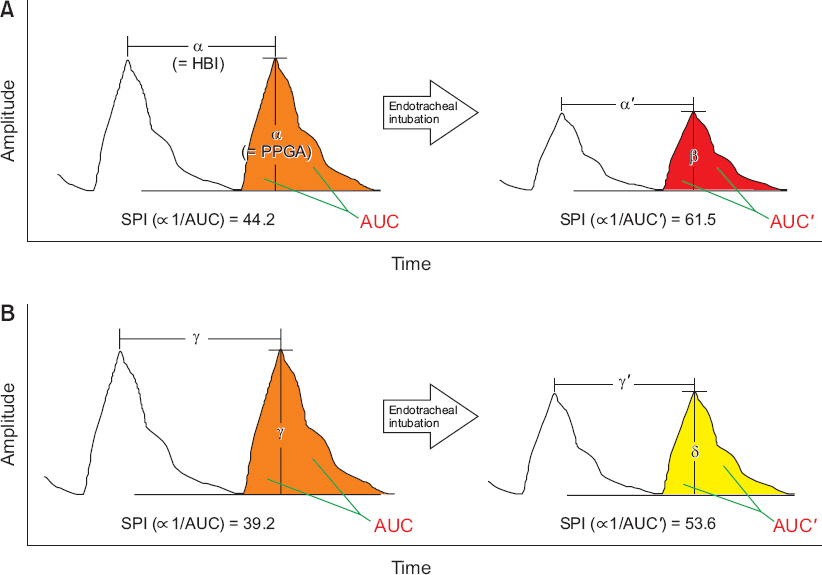
In summary, Park et al. [19] reported that for children undergoing tonsillectomy under general anesthesia, intraoperative fentanyl requirement was lower for the SPI-guided analgesia group than for hemodynamic parameters-based analgesia, but postoperative pain and emergence agitation scores and rescue fentanyl dose were higher in the SPI-guided analgesia group, and thus SPI values in children are less likely to accurately reflect the nociception-antinociception balance due to their unique cardiovascular structure and physiology. It was concluded that re-establishment of the target SPI range or the development of new formulas for children is necessary for proper intraoperative analgesia in children undergoing general anesthesia.
Ledowski et al. [24] also found that SPI cut-off values of 50, the most commonly used cut-off value in SPI-related studies, do not have clinically appropriate sensitivity or specificity to predict the presence of acute pain in the recovery room, and suggested that it may be more appropriate to adjust to a cutoff of 40 or lower (which is lower than the existing cut-off value of 50) to provide intraoperative analgesia that avoids moderate and severe postoperative pain in children. Ledowski et al. [24] reaffirmed that, in children, intraoperative analgesia during general anesthesia should be performed based on SPI values that differ from those in adults.
Song et al. [25] also reported there was no significant difference in SPI changes during cranial pin fixation among the groups received sufentanil infusion at three different rates (0.2, 0.5, and 0.8 μg/kg/h) in pediatric patients aged 2-12 years who underwent cranial pinning under general anesthesia, suggesting that SPI may not be sensitive enough to monitor a level of nociception and analgesia in children.
A systematic review using meta-analysis of six randomized controlled trials, examining the efficacy and safety of SPIguided analgesia and conventional analgesia based on hemodynamic parameters (blood pressure and HR) and during surgery under general anesthesia, showed that SPI-based analgesia reduced intraoperative opioid requirement and shortened extubation time, and there was no difference in the degree of postoperative pain and the incidence of perioperative complications [32]. Based on these findings, the systematic review suggested that nociception monitoring and analgesic administration using SPI are more useful for providing proper analgesia under general anesthesia in various clinical situations. However, considering that the number of randomized controlled studies included in this study is few, and only one of the included studies is for children [19], and the results of other studies related to SPI in children are conflicting [24-26], further studies are needed to determine the efficacy of SPI for intraoperative analgesia, especially in children.
NOCICEPTION MONITORING TOOLS USING HEART RATE VARIABILITY
Heart rate variability (HRV)
HRV refers to the degree of change (variability) in time and frequency analysis of the interval between successive heartbeats, which reflects the activity and balance of the autonomic nervous system, i.e., the interactions between the sympathetic and parasympathetic nervous systems. HRV monitoring devices calculate and measure HRV parameters such as standard deviations of normal RR intervals on standard electrocardiogram (time-domain analysis) or high-frequency (HF), low-frequency (LF), very low-frequency (VLF) power, and LF/HF ratio (frequency domain [power spectral density] analysis) quickly, easily, and non-invasively [33,34]. The LF/HF ratio is a quantitative measure of the overall balance between the sympathetic and parasympathetic nervous system and, when the ratio is high, indicates an increase in sympathetic activity or inhibition of parasympathetic activity.
HRV can be measured in both conscious patients and those under sedation or anesthesia [35-37], but similar to SPI, it can be affected by several physiological or psychological conditions such as age (typically decreasing with age) [38], drugs [39], psychological problems [40], comorbidities [41,42], depth of sedation or anesthesia [43,44], and surgical stimuli [45]. Several clinical studies examining the correlation between the intensity of noxious stimuli such as surgical stimuli and corresponding HRV in anesthetized adult patients suggested that HRV could be used as an objective pain assessment tool [46,47]. In contrast, studies for HRV in unanesthetized adult patients showed conflicting results [48,49].
In addition, numerous studies have investigated the relationship between established behavioral indicators of pain and HRV at the evaluation of acute pain in pediatric patients, including preterm infants, newborn (neonate) infants, infants, and children in various clinical settings [50-52]. Consequently, HRV usually responded well to pain in most pediatric age groups, but the findings in infants were inconsistent [53]. On the other hand, physiological indicators (nociception monitoring devices), including HRV, are the only tools that can measure nociception in anesthetized younger children, but the results reported so far have limitations due to the nature of HRV, which is susceptible to several factors [54,55].
Therefore, several real-time algorithms and indices using them have been developed to correct such confounding factors of HRV so that changes in HRV correspond well to the intensity of nociception. These algorithms and indexes include analgesia nociception index, cardiorespiratory coherence, and newborn infant parasympathetic evaluation (NIPE).
Analgesia nociception index (ANI)
ANI (MetroDoloris Medical Systems, France) combines electrocardiogram and respiratory rate with high-frequency adjustment (0.15-0.4 Hz) in the frequency domain analysis of HRV and shows parasympathetic activity in the numerical range of 0 (maximal pain) to 100 (no pain) (as opposed to SPI). Previous studies on ANI regarded ANI ≥ 50 as appropriate analgesia and predicted that ANS responsiveness was caused by nociceptive stimulation when ANI < 30 [56,57].
The efficacy of ANI in intraoperative nociception monitoring and analgesia has been well documented through studies performed in adults [56,58,59]. Recent studies in pediatric patients also reported that ANI was more sensitive to detecting surgical stimuli during surgery and useful for monitoring intraoperative analgesia than other hemodynamic parameters [60-62].
Considering that ANI is shown to reflect not only intraoperative pain but also postoperative pain, that is, the degree of pain in conscious patients, it may have advantages over other pain monitoring devices [63,64]. Nevertheless, there are still studies that reported the opposite results [57,65], much like the controversial issue of its reliability in conscious patients in the correlation between HRV and pain intensity [48,49].
Cardiorespiratory coherence
Cardiorespiratory coherence, a non-invasive nociception monitoring device, measures a degree of ANS activity by analyzing the linear combination intensity between heart rate and respiration during general anesthesia and assesses the degree of pain in a range from 0 (low coherence, strong nociception) to 1 (high coherence, no nociception). It includes real-time cardiorespiratory coherence; cardiorespiratory coherence algorithm, or wavelet transform cardiorespiratory coherence algorithm. Although there are very few studies evaluating the efficacy of cardiorespiratory coherence to date, most of them have been conducted in children, and they reported notable outcomes that cardiorespiratory coherence is more sensitive and superior to hemodynamic parameters in detecting nociception, antinociception, and movement in pediatric patients undergoing general anesthesia [66-68].
Newborn infant parasympathetic evaluation (NIPE)
ANI was developed for HRV analysis in children over 2 years old and adults. Newborns, infants, and children under 2 years of age require a modified approach to HRV analysis because they have a lower HRV due to an immaturity of the ANS and high basal heart rate. The NIPE index (MetroDoloris Medical Systems), a modified form of ANI nociception monitoring, was developed for use in newborns (including premature infants) and children under 2 years of age [69]. The basic principle of NIPE is to analyze the parasympathetic activity of the ANS in real-time using HRV analysis. HRV signals above 0.15 Hz allow for automated HRV analysis of data indicative of parasympathetic nervous system activity via a high pass filter and exhibiting physiological respiratory sinus arrhythmia [69]. This automated analysis is quantified by the NIPE index, which ranges between 0-100 to reflect relative parasympathetic activity, with higher values indicating higher levels of parasympathetic activity. A NIPE index of less than 50 in newborns and infants under anesthesia usually suggests a presence of stress or nociception and insufficient analgesia [70].
NIPE does an excellent job reflecting a balance of nociception and antinociception. NIPE decreases in the presence of nociception in anesthetized infants and newborns, while increases in cases of loss of nociception or administration of analgesics [70,71]. In addition, Valencia-Ramos et al. [72] reported that NIPE monitoring reflected a change in the degree of comfort during nebulization for conscious infants with bronchitis in the intensive care unit. NIPE was suggested as a comfort monitoring system for infants. NIPE may also reflect psychological conditions such as psychological stability or discomfort and stress in conscious infants and newborns. Taken together, even if the evidence is still lacking, NIPE monitoring of nociception and analgesia, or discomfort and comfort in newborns and infants under two years of age under anesthesia or awareness may be more effective than other nociception monitoring modalities. However, Cremillieux et al. [73] reported that the NIPE index during painful procedures of premature infants in the neonatal intensive care unit did not reliably reflect acute pain. Thus, further research is required to study these issues.
NOCICEPTION MONITORING TOOLS USING SKIN SWEATING
Skin conductance algesimeter (SCA)
A SCA measures an increase in stress reflected in changes in the activity of the sympathetic nervous system. When the sympathetic nerves are activated, the plantar sweat glands in the palms and soles are filled with sweat. When the sweat reaches the skin, the skin resistance decreases, and the skin conduction increases, and when the sweat is reabsorbed, the skin conduction decreases again. When the skin nerves are activated by stimulation, both amplitude and frequency of efferent skin nerve bursts increase. Therefore, the increase in the number of skin conductance fluctuations (NSCF) and amplitude of skin conductance fluctuations in SCA measurements may be interpreted as increased activity of the sympathetic nervous system [74,75].
Unlike other nociception monitoring tools, the SCA index is not affected by circulatory changes, cardiac activity, vasoactive drugs, or neuromuscular blockade. Therefore, SCA is more sensitive and specifically associated with pain and noxious stimuli [76]. The SCA index responds quickly (in seconds) and allows continuous and objective monitoring with a wide range of indications in various clinical situations. It also has higher sensitivity and specificity in assessing pain than other monitoring devices currently available. SCA has been extensively studied, particularly in pediatric patients, for its efficacy and is known to be useful in assessing pain and analgesia. For premature infants, SCA is more sensitive and specific than behavioral-state observations in evaluating heel stick pain during blood sampling, tactile stimulation stress, and high decibel sound stimuli [77-79]. Both in healthy newborns and artificially ventilated infants, an increased NSCF on SCA monitoring correlated well with occurrences of pain and discomfort [80,81].
Several studies evaluating the efficacy of SCA concerning general anesthesia in adult patients have shown that the SCA index is useful in monitoring perioperative stress with increasing values during nociceptive stimulation, such as endotracheal intubation and tetanic stimulation, and decreasing values during analgesic infusion [82,83]. Regarding postoperative pain, several studies have reported that the SCA index is well correlated with the numeric rating scale assessed by the recovery room [84,85].
Few studies of SCA have been conducted on children under general anesthesia. Sabourdin et al. [61] reported that the responses of SCA to remifentanil at different infusion rates in children under general anesthesia was less sensitive than those of ANI. Therefore, despite many advantages of SCA, the evidence is still lacking in its efficacy for analgesia in pediatric patients undergoing general anesthesia. Thus further studies are required on these topics.
NOCICEPTION MONITORING TOOLS USING PUPILLARY CHANGE
Pupillometry
Pupillometry is based on the evaluation of the pupillary reflex dilatation induced by nociceptive stimulation. Pupillometry is a non-invasive monitoring technique which measures dynamic pupillary diameter by an infrared camera. Pupillary diameter increases in response to nociceptive stimulation.
The degree of pupillary dilatation in patients with anesthesia using propofol or inhalational anesthetics is associated with the intensity of nociceptive stimuli during operation. The pupillary reflex dilatation by nociception in pediatric patients was maintained during deep sedation with ketamine, and the degree of pupillary dilatation was related to the intensity of the nociceptive stimuli [86]. Therefore, pupillometry can be a useful nociception monitoring tool in pediatric patients sedated with ketamine. Additionally, it has been suggested that pupillometry is a useful nociception monitoring device by other studies in children and adolescents undergoing general anesthesia, which showed that nociceptive stimuli during anesthesia cause pupillary reflex dilatation, and administration of opioid analgesics or deep anesthesia (hypnosis) decreases the pupil diameter [87-89]. However, it must be considered that other factors such as severe anxiety [90] or drugs [91,92] also influence the pupil diameter and reflex.
REGIONAL ANALGESIA AND OPIOID CONSUMPTION DURING GENERAL ANESTHESIA
Regional analgesia in pediatrics are performed after the induction of general anesthesia and before skin incision to reduce the surgical stress response and to spare intravenous opioid administration during surgery. The adequacy of regional analgesia in children has traditionally been assessed by monitoring changes in hemodynamic parameters caused by noxious stimuli. Inadequate regional analgesia is often defined as an increase in heart rate of about 10-20% or more from baseline within 1 to 2 minutes after surgical incision [93,94]. However, the use of these hemodynamic parameters to assess the effects of regional analgesia is not standardized and may be inaccurate [95-98]. Therefore, there have been attempts to perform a faster and more accurate evaluation and to minimize intravenous opioid requirements during surgery by determining the effects of regional analgesia using nociception monitoring devices that are proven effective in monitoring the nociception-antinociception balance during surgery.
As previously mentioned, Harju et al. [26] reported that no increase in SPI was observed during surgical incision, and the responsiveness of SPI to noxious stimuli was blunted in children undergoing ultrasound-guided ilioinguinal and iliohypogastric nerve block after anesthesia induction for inguinal hernia surgery, suggesting that SPI monitoring may be useful in discriminating the success of regional analgesia in pediatric patients. Song et al. [99] observed significant changes in some HRV parameters after performing a caudal block in pediatric patients undergoing urological surgery under general anesthesia. They concluded that these HRV changes indicated reduced sympathetic activity and increased heart rate predictability, suggesting that the assessment of the HRV parameters can be an indicator of a successful caudal block. Migeon et al. [93] assessed pupillary reflex dilatation and ANI monitoring to evaluate the adequacy of regional analgesia while performing a neuraxial or peripheral nerve block after anesthesia induction in pediatric patients undergoing urological and orthopedic surgeries under sevoflurane anesthesia. In patients with a failed nerve block, the pupil diameter increased significantly, and ANI decreased within 1 min. Therefore, it was suggested that these two nociception monitoring tools would be helpful in rapidly and accurately discriminating the success of regional analgesia in pediatric patients. Meanwhile, in a prospective randomized trial performed by Dundar et al. [100], after performing a thoracic paravertebral block in adult patients undergoing breast surgery under general anesthesia, the rate of remifentanil infusion was adjusted based on ANI monitoring (maintained at 50-70) in an experimental group for further analgesia during surgery. As a result, it was reported that intraoperative remifentanil consumption in the experimental group based on ANI was significantly lower than that of the control group based on hemodynamic parameters, suggesting that ANI monitoring may help optimize opioid consumption during surgery in these clinical settings.
Comprehensively considering these findings, further studies are needed in pediatric patients with various regional analgesia and nociception monitoring devices in various clinical situations.
CONCLUSIONS
Nociception monitoring tools using ANS activity have been developed in several ways. Each monitoring device evaluates sympathetic or parasympathetic tone changed by nociception and the monitoring devices share being more effective than conventional hemodynamic parameters. At the same time, depending on which autonomic surrogate marker is used, the basic operating principle and characteristics of the monitoring device differ, and their usefulness in various clinical situations also differs. Therefore, a comprehensive analysis and understanding of these principles and study results are needed. This review analyzes the efficacy and limitations of several nociception monitoring devices, mainly focusing on the intraoperative analgesia in pediatric patients under general anesthesia and additionally comparing details about clinical conditions such as arousal state, postoperative pain, regional analgesia, and includes adult or younger children (newborn or infant) populations when needed.
Although most of the nociception monitoring devices still lack studies with pediatric patients, most nociception monitors in children (as in adults) appear to be more useful than the standard clinical practice that uses hemodynamic parameters in the evaluation and treatment of intraoperative nociception during general anesthesia.
Summarizing the characteristics of each monitoring device, SPI seems to be less valid and limited for children than for adults. It may be necessary to apply a lower target value range (maintained below 40) or to develop new formulas fitted to children to provide adequate analgesia during surgery under general anesthesia in children. ANI has shown promising results in anesthetized adults, and recently positive results along with cardiorespiratory coherence have been reported in pediatric patients. Especially, NIPE is expected to be useful for providing adequate analgesia in newborns and infants and children under 2 years of age under anesthesia or a conscious state. SCA may be best used to assess stress in conscious or sedated newborns and younger children, but there is still little evidence of the efficacy of the analgesia during general anesthesia in pediatric patients. Pupillometry has shown reliable results in pediatric patients under anesthesia, as in adults, but has some pitfalls, because the measurement may be inaccurate or complicated by a range of factors. It has been reported that ANI performs well compared to other devices on postoperative pain, but there are still suboptimal results. SPI, ANI, and pupillometry may be useful for evaluating the effects of regional analgesia performed during general anesthesia. In this situation, ANI also enables the optimization of opioid consumption.
A thorough understanding of the pros and cons of the nociception monitoring tools summarized above and their application in clinical situations will provide a more effective and safe intraoperative analgesia for pediatric patients undergoing general anesthesia, and it may also facilitate the planning and conduct of research on the use of intraoperative nociception monitoring in pediatric patients.










