|
 |
- Search
| Anesth Pain Med > Epub ahead of print |
Abstract
Background
Opioids administered as bolus doses or continuous infusions are widely used for major and daycare surgeries. Opioid-free anesthesia is multimodal anesthesia and analgesia that does not use opioids, benefiting patients from opioid-related adverse effects. We compared the postoperative analgesic requirements of patients scheduled for elective laparoscopic cholecystectomy under opioid-free and opioid-based anesthesia.
Methods
Study included 88 patients aged 18-60 years with American Society of Anesthesiologists physical status 1 and 2 who underwent elective laparoscopic cholecystectomy. Participants were randomly divided into two groups with 44 in each. The opioid-free group was administered an intravenous bolus of ketamine and dexmedetomidine, whereas fentanyl was used in opioid group. Primary outcome was to compare the total amount of fentanyl consumed by both groups during 6 h postoperative period. Episodes of postoperative nausea and vomiting (PONV) and vital signs were noted throughout the postoperative period to analyze the secondary outcomes.
Results
Both groups had similar demographic characteristics. The opioid-free group required lesser analgesia within the first 2 h (61.4 ± 17.4 vs. 79.0 ± 19.4 of fentanyl, P < 0.001), which was statistically significant. However, fentanyl consumption was comparable between the groups at 6 h (152 ± 28.2 vs. 164 ± 33.4, P = 0.061). Compared with 4.5% of the participants in the opioid-free group, 34% of those in the opioid-based group developed moderate PONV.
Anesthesia requires a full spectrum of drugs, from which an anesthetic plan can be implemented to achieve the desired level of sedation, analgesia, amnesia, muscle relaxation, and reflex abolition. Opioid administration as a bolus dose or continuous infusion is widely used by anesthesiologists in major and day care surgeries. The use of opioids during anesthesia is associated with various opioid-related adverse effects such as respiratory depression, opioid-induced hyperalgesia, nausea and vomiting, urinary retention, paralytic ileus, and the risk of cognitive and sleep dysfunction [1-4]. This negative side effect profile of opioids may cause delayed recovery and discharge of patients from the post-anesthesia care unit as well as unanticipated hospital readmissions.
The emergence of opioid-free anesthesia was triggered by the adverse effects of opioids and the ongoing opioid epidemic, which began in high-income countries, such as the United States, in the 1990s and has now expanded to Europe and Asia [5,6]. It is a multimodal anesthetic and analgesic without the use of opioid drugs and can play a significant role in enhanced recovery after surgery [7]. Drugs useful for opioid-free anesthesia include dexmedetomidine, ketamine, dexamethasone, lignocaine, non-steroidal anti-inflammatory drugs, paracetamol, and beta-blockers such as esmolol, benzodiazepines, and magnesium sulfate [8-13]. The use of opioids after abdominal surgery is recommended only when non-opioid drugs provide insufficient analgesia.
Thus, there is a need to study and evaluate new non-opioid pain medications after laparoscopic cholecystectomy as part of an opioid reduction strategy. Dexmedetomidine is a centrally acting alpha 2 adrenoreceptor agonist with a good potential for improving analgesia, hemodynamic responses to endotracheal intubation, and hemoperitoneum creation. Its perioperative analgesic potential and sedative properties have been widely studied in different types of major surgeries, with promising results [14]. The role of dexmedetomidine in early recovery after day care surgery is a new area of interest among anesthesiologists. Ketamine has excellent analgesic, amnesic, and opioid-sparing properties. In small doses (doses < 0.5 mg/kg), it has good analgesic effects with minimal side effects [15,16]. A single dose of ketamine can significantly reduce postoperative opioid consumption to a great extent in day care surgeries, such as laparoscopic cholecystectomy.
Thus, this randomized controlled trial was designed to compare the postoperative analgesic requirements of adult patients undergoing elective laparoscopic cholecystectomy under opioid-free anesthesia with dexmedetomidine and ketamine versus conventional opioid-containing anesthesia. We hypothesized that opioid-free anesthesia would provide better postoperative analgesia than opioid-based anesthesia in elective laparoscopic cholecystectomy.
This prospective, randomized, double-blind, controlled clinical trial was conducted at the All India Institute of Medical Sciences, Patna, India. This study was approved by the institutional ethics committee (AIIMS/Pat/IEC/PGTh/July 20/06 dated 20/09/2021) and registered with the Clinical Trials Registry, India (CTRI/2022/02/039883). This study included adult patients scheduled for elective laparoscopic cholecystectomy belonging to American Society of Anesthesiologists physical status 1 and 2 in the age group of 18-60 years with a body mass index (BMI) of 18-35 kg/m2. Patients with a BMI > 35 kg/m2; pregnant and breastfeeding women; a history of hepatic, renal, or cardiac disease; alcohol abuse; drug abuse; psychiatric illness; and a history of chronic pain (pain present for more than 6 months) were excluded from the study. Each patient was assigned a computer-generated number and randomized into two groups in a 1:1 ratio. Randomization was block randomization with a block size of four. The allocation was concealed using an opaque sealed envelope. Opioid-free group patients were administered dexmedetomidine infusion at a rate of 0.5 mcg/kg/h, initiated 10 min before induction, and ketamine IV bolus dose of 0.35 mg/kg was administered before skin incision. Dexmedetomidine infusion was stopped at the time of removal of the gall bladder. The opioid-based group was administered fentanyl intravenous bolus dose of 2 mcg/kg before induction and intermittent doses of 0.5 mcg/kg were administered intraoperatively if the mean arterial pressure (MAP) and heart rate (HR) were greater than ± 20% of the baseline value.
All patients in both groups were administered midazolam (0.03 mg/kg) before surgery. Patients in both groups received injections of dexamethasone (8 mg) and diclofenac (75 mg) before induction. Preoxygenation of the patients was performed before the induction of anesthesia. Anesthesia was induced using propofol (1.5-2.5 mg/kg) intravenously. Muscle relaxation was attained using atracurium (0.5 mg/kg) intravenously. Endotracheal intubation was performed in all patients. Muscle relaxation was maintained with a bolus dose of atracurium. Patients were ventilated using a mixture of sevoflurane, oxygen, and air titrated to a bispectral index of 40-60. The port sites were infiltrated with 2% lignocaine. After surgery, muscle relaxation was reversed with neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg). Paracetamol (1,000 mg) and ondansetron (4 mg) were administered immediately after removal of the gall bladder. Diclofenac (75 mg every 12 h) and paracetamol injection (1,000 mg) was prescribed for postoperative analgesia. A patient-controlled analgesia (PCA) pump containing 100 ml solution of 10 mcg/ml fentanyl was set to deliver an intermittent IV bolus dose of 25 mcg at a lockout interval of 15 min, without any background infusion and was administered as a rescue analgesic in both groups. The primary objective was to compare the cumulative amount of fentanyl used for pain relief in the first 6 h after extubation in adult patients undergoing elective laparoscopic cholecystectomy.
The secondary objectives were to assess postoperative pain scores using a numerical rating scale at 2, 6, 12, and 24 h; find the total amount of intravenous fentanyl consumption at 2, 12, and 24 h postoperative periods; estimate the time taken for the first use of rescue analgesia; and assess the incidence of postoperative nausea and vomiting (PONV) episodes experienced by the patients in the first 24 h postoperative period among patients in the opioid-free and opioid-containing groups. Postoperative pain severity was assessed using an 11-point numeric rating scale with scores ranging from 0 to 10, with 0 indicating no pain and 10 indicating worst pain. PONV were assessed using a 4-point verbal rating scale (VRS): none, mild, moderate, and severe. Other opioid-related adverse effects such as respiratory depression, constipation, and itching were also assessed.
The sample size was calculated at an alpha risk of 0.05; 39 patients per group provided 80% power and detected a 40% reduction in fentanyl consumption in the treatment group based on a previous study by Bakan et al. [17] Assuming a dropout rate of 10%, the total sample size was calculated as 43 per group and rounded off to 44 per group (88 total sample sizes).
Data were expressed as numbers, percentages, or mean ± standard deviation. Normality was assessed using a quantile-quantile plot. Chi-square or Fisher’s exact tests were used to compare categorical data, as appropriate. Independent Student’s t-test or Mann-Whitney U test, if necessary, was used to compare quantitative variables. Continuous data were analyzed using independent Student’s t-test for significance. Statistical significance was set at P < 0.05. Statistical data were analyzed using Jamovi 2.3.18 software (The Jamovi Project 2022).
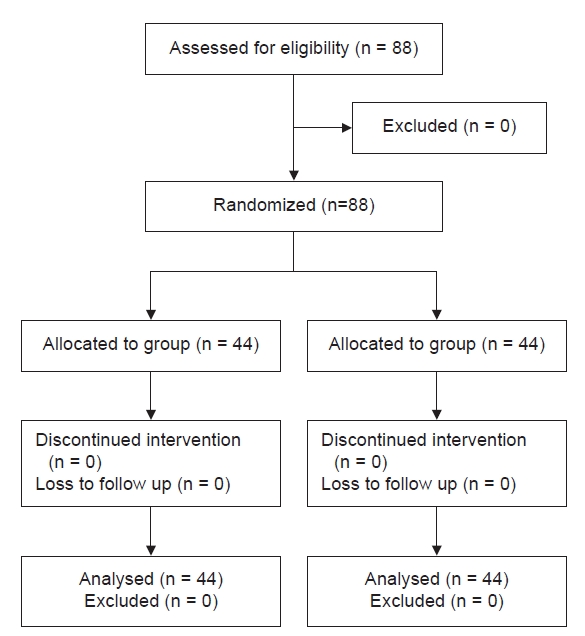
A total of 88 patients who fulfilled the inclusion criteria participated in the study and there were no dropouts. Fig. 1 shows a consort-flow diagram of the patient selection, allocation to each group, follow-up, and study analysis. The demographic characteristics of the patients were comparable between the groups (Table 1). Postoperative fentanyl consumption during the first 6 h postoperative period was 152 ± 28.2 mcg in the opioid-free group and 164 ± 33.4 mcg in the opioid-based group, which did not show any statistically significant difference. There was no statistically significant difference in the total amount of fentanyl consumed between the two groups at the 12 and 24 h postoperative periods; however, a significant difference was observed in the first two hours of the postoperative period (Table 2). Pain scores also showed a statistically significant difference 2 h postoperatively. The time taken for the first use of the PCA was significantly longer in the opioid-free group, which showed better analgesia during the immediate postoperative period (Table 3). The VRS for PONV showed a higher number of patients with moderate PONV in the opioid-based group: 34% of the patients in the opioid-based group had moderate PONV compared with 4.5% of those in the opioid-free group (Table 4). Intraoperative hemodynamic parameters were not significantly different between the two groups. However, when comparing the HR and MAP readings from baseline, the opioid-free group had higher values than the opioid-based group (Fig. 2). The variations in hemodynamic parameters during pneumoperitoneum were similar in both the groups (Fig. 3).
The findings of this study indicate that patients who were administered opioid-free anesthesia with ketamine and dexmedetomidine had a reduction in fentanyl requirement 2 h after extubation compared with those of the opioid-based group. Although no intraoperative fentanyl was used in the opioid-free group, patients in both groups had a comparable fentanyl requirement in the first 6 h of the postoperative period. In our study, the time to first rescue analgesia was also significantly longer in the opioid-free group than in the opioid group.
In recent years, non-opioid anesthetic techniques have gained recognition for reducing opioid-related adverse effects and for improving recovery. Owing to its anxiolytic, analgesic, and sympatholytic properties, dexmedetomidine has been used as an opioid substitute in numerous surgical interventions. We also administered a sub-anesthetic dose of intravenous ketamine to the opioid-free group because it provides effective analgesia without compromising clinical safety. Combining dexmedetomidine and ketamine with other non-opioid analgesic medications using a multimodal approach can produce synergistic analgesia. The clinically significant difference in pain scores in the 2 h postoperative period in the opioid-free group could be due to the extension of the analgesic and sedative effects of dexmedetomidine and ketamine during the initial hours of the postoperative period. The duration of action of dexmedetomidine and ketamine is up to 90 min after intravenous administration; therefore, during this period, patients who underwent dexmedetomidine ketamine anesthesia had better postoperative analgesia and pain scores than those who were administered opioid anesthesia.
A randomized controlled trial conducted by Bharadwaj et al. [18] on laparoscopic urological procedures in patients with obesity supported our findings. They found that opioid-free anesthesia provided superior analgesia compared with opioid-based anesthesia and that rescue analgesic consumption was significantly lower in the first 24 h of the postoperative period. They used a combination of dexmedetomidine, ketamine, and lignocaine in their study in comparison with fentanyl-based anesthesia. Compared with our findings in this study, the opioid-free group experienced a prolonged analgesic effect, which may have been due to the administration of an additional drug, lignocaine (1.5 mg/kg), at induction, followed by 0.1 mg/kg/h of lignocaine infusion.
PONV in the first 24 h of the postoperative period were assessed as secondary outcomes in each group. For this purpose, a simple VRS for nausea and vomiting was used. In this study, severe nausea was not reported in any of the groups; however, the incidence of mild-to-moderate PONV was significantly higher in the opioid group. PONV are known side effects of opioid use; therefore, the decreased incidence of PONV in the study group may have been related to the avoidance of intraoperative opioids. Ziemann-Gimmel et al. [19] found similar results, demonstrating that opioid-free total intravenous anesthesia was associated with a substantial reduction in the relative risk of PONV compared with balanced anaesthesia. They reported that PONV were significantly more severe in the opioid group; however, there was no significant difference in the number of patients requiring rescue antiemetic medication or in the number of doses required.
The results of our study indicated that during the intraoperative and postoperative periods, the mean (HR) was statistically comparable in both groups, except immediately after intubation, where the HR was higher in the opioid-free group. No significant difference was found between the groups when MAP was compared. The increase in the HR was probably due to the intubation response because we used a fixed infusion rate of dexmedetomidine and did not provide a loading dose. In contrast to our findings, Beloeil et al. [20] reported a greater incidence of bradycardia and five cases of severe bradycardia in the dexmedetomidine group. In some cases, bradycardia occurred during carbon dioxide insufflation during laparoscopic surgery. They observed a high incidence of severe bradycardia due to a higher dose of dexmedetomidine infusion; in one case, it was related to an incorrect estimation of the patient’s weight. We did not observe any cases of severe bradycardia, which may be explained by the fact that we administered dexmedetomidine at a fixed low dose and avoided bolus administration. In addition, IV ketamine may have provided hemodynamic stability by counteracting bradycardia. Further research is needed to fully understand the hemodynamic effects of the combination of dexmedetomidine and ketamine as we did not explore hemodynamics as the primary outcome. We did not observe any other opioid-related complications in the opioid or study groups.
Our study had some limitations. The exclusion of patients in the ASA categories 3 and 4 reduced the generalizability of the trial. As there was a lack of information regarding the hemodynamic effects of the drug combinations used in these categories, these categories were excluded. We observed postoperative pain for 24 h only, which may be insufficient to determine the long-term effects. A more comprehensive understanding of the effects on postoperative pain can be attained by obtaining data over a longer period. A four-point VRS was used to measure PONV symptoms, although this scale has not been validated for assessing PONV. However, we found that patients could easily comprehend this scale and found it simple to understand.
We concluded that administering dexmedetomidine and ketamine together in opioid-free anesthesia for patients undergoing elective laparoscopic cholecystectomy reduced opioid consumption in the first 2 h of the postoperative period and prolonged the time to the first use of rescue analgesia. The opioid-free anesthesia technique was also associated with decreased PONV. Thus, this anesthetic technique may be used as an alternative for selected patients undergoing elective laparoscopic cholecystectomy.
Notes
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Kunal Singh. Writing - review & editing: Vishnuraj K R, Nishant Sahay, Chandni Sinha, Amarjeet Kumar, Neeraj Kumar. Conceptualization: Vishnuraj K R, Kunal Singh, Nishant Sahay. Formal analysis: Vishnuraj K R, Kunal Singh, Nishant Sahay, Chandni Sinha, Amarjeet Kumar, Neeraj Kumar. Visualization: Kunal Singh. Validation: Kunal Singh, Chandni Sinha, Amarjeet Kumar, Neeraj Kumar.
Fig. 2.
Comparison of the mean heart rate between the two groups during the intraoperative and 24 postoperative periods.

Fig. 3.
Comparison of mean arterial pressure between the two groups during the intraoperative and 24 postoperative periods.
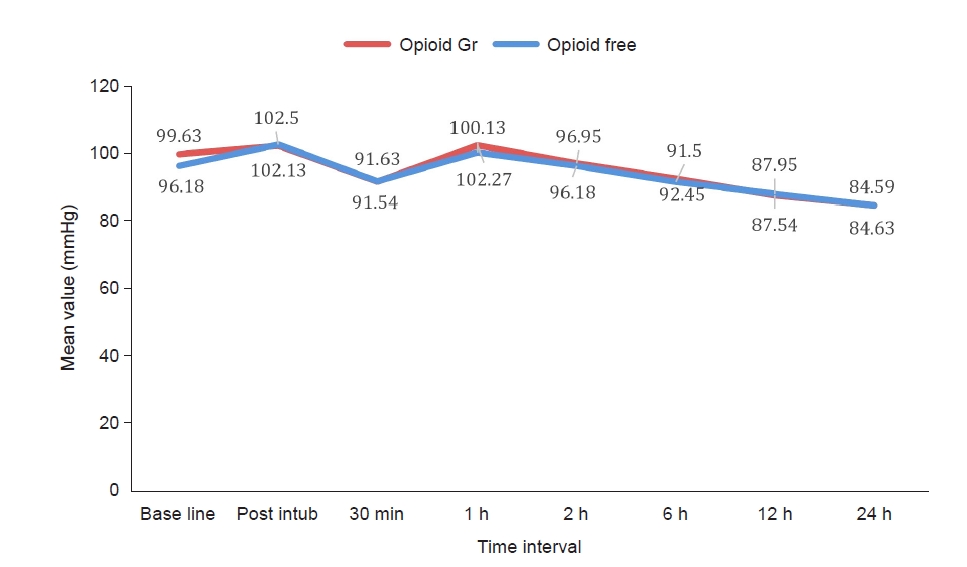
Table 1.
Demographic Data of the Patients and Duration of Surgery
| Parameters | Opioid‑free group (n = 44) | Opioid‑based group (n = 44) | P value |
|---|---|---|---|
| Age (yr) | 36.0 ± 10.4 | 40.3 ± 11.3 | 0.073* |
| Gender (F/M) | 31/13 | 32/12 | 0.813† |
| Weight (kg) | 56.9 ± 10.14 | 58.8 ± 9.42 | 0.346 |
| BMI (kg/m2) | 21.6 ± 2.91 | 22.2 ± 2.98 | 0.323 |
| Duration of surgery (min) | 48.9 ± 11.7 | 51.6 ± 13.0 | 0.303* |
Table 2.
Postoperative Fentanyl Requirement and Time to First Rescue Analgesic
| Outcome | Opioid‑free group (n = 44) | Opioid‑based group (n = 44) | Mean difference (95% CI) | P value |
|---|---|---|---|---|
| Postoperative fentanyl consumption* (µg) | ||||
| 2 h | 61.4 ± 17.4 | 79.0 ± 19.4 | -17.61 (-25.4 to -9.79) | 0.001 |
| 6 h | 152 ± 28.2 | 164 ± 3.34 | -12.50 (-25.6 to 0.61) | 0.061 |
| 12 h | 204 ± 38.5 | 217 ± 33.1 | -13.07 (-28.3 to 2.15) | 0.091 |
| 24 h | 235 ± 44.2 | 242 ± 37.6 | -7.39 (-24.8 to 10.01) | 0.401 |
| Time taken for first use of PCA (min) | 17.9 ± 4.69 | 11.2 ± 2.90 | 6.68 (5.03 to 8.33) | 0.001 |
Table 3.
Analysis of postoperative pain scores
| Outcome | Opioid‑free group (n = 44) | Opioid‑based group (n = 44) | P value |
|---|---|---|---|
| NRS scores* | |||
| 2 h | 5.0 (4, 6) | 5.0 (4, 6) | 0.001 |
| 6 h | 3.0 (2, 4) | 3.0 (2, 4) | 0.342 |
| 12 h | 2.0 (1, 3) | 2.0 (1, 3) | 0.974 |
| 24 h | 1.0 (1, 2) | 1.0 (1, 2) | 0.646 |
Table 4.
Incidence of PONV
| PONV severity | Opioid‑free group (n = 44) | Opioid based group (n = 44) | P value |
|---|---|---|---|
| Mild | 42 (95.5) | 29 (65.9) | |
| Moderate | 2 (4.5) | 15 (34.1) | 0.001* |
| Severe | 0 | 0 |
REFERENCES
1. Nagappa M, Weingarten TN, Montandon G, Sprung J, Chung F. Opioids, respiratory depression, and sleep disordered breathing. Best Pract Res Clin Anaesthesiol 2017; 31: 469-85.


2. Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and meta-analysis. Br J Anaesth 2014; 112: 991-1004.

3. Roberts GW, Bekker TB, Carlsen HH, Moffatt CH, Slattery PJ, McClure AF. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg 2005; 101: 1343-8.


4. Dinges HC, Otto S, Stay DK, Bäumlein S, Waldmann S, Kranke P, et al. Side-effect rates of opioids in equianalgesic doses via intravenous patient-controlled analgesia: a systematic review and network meta-analysis. Anesth Analg 2019; 129: 1153-62.


5. Kuehn BM. Scientists, officials eye tools aimed at combating abuse of painkillers. JAMA 2012; 307: 19-21.


6. Casati A, Sedefov R, Pfeiffer-Gerschel T. Misuse of medicines in the European Union: A systematic review of the literature. Eur Addict Res 2012; 18: 228-45.



7. Tan M, Law LSC, Gan TJ. Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can J Anesth 2015; 62: 203-18.



8. Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anesth 2006; 53: 646-52.



9. Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology 2010; 113: 639-46.



10. Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side effects: a systematic review and meta-analysis. Br J Anaesth 2013; 110: 191-200.

11. Weibel S, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: Aa systematic review with trial sequential analysis. Br J Anaesth 2016; 116: 770-83.

12. Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg 2010; 110: 1170-9.

13. Collard V, Mistraletti G, Taqi A, Asenjo JF, Feldman LS, Fried GM, et al. Intraoperative esmolol infusion in the absence of opioids spares postoperative fentanyl in patients undergoing ambulatory laparoscopic cholecystectomy. Anesth Analg 2007; 105: 1255-62.


14. Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 2012; 116: 1312-22.

15. Brinck EC, Tiippana E, Heesen M, Bell RF, Straube S, Moore RA, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev 2018; 12: CD012033.


16. McCartney CJ, Sinha A, Katz J. A qualitative systematic review of the role of N-methyl-D-aspartate receptor antagonists in preventive analgesia. Anesth Analg 2004; 98: 1385-400.


17. Bakan M, Umutoglua T, Topuza U, Uysala H, Bayram M, Kadioglu H, et al. [Opioid free total intravenous anesthesia with propofol, dexmedetomidine and lidocaine infusions for laparoscopic cholecystectomy: a prospective, randomized, double blinded study]. Rev Bras Anestesiol 2015 65: 191-9. Portuguese.


18. Bhardwaj S, Garg K, Devgan S. Comparison of opioid-based and opioid-free TIVA for laparoscopic urological procedures in obese patients. J Anaesthesiol Clin Pharmacol 2019; 35: 481-6.



- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others