|
 |
- Search
| Anesth Pain Med > Epub ahead of print |
Abstract
Background
Mounier-Kuhn syndrome (MKS) is a rare disorder characterized by abnormal dilation of the trachea and main bronchi. MKS can be easily missed on chest X-rays, making diagnosis difficult. Under general anesthesia, challenges such as airway leakage or collapse during mechanical ventilation may complicate the achievement of adequate tidal volumes.
Case
A 94-year-old woman requiring emergency hemiarthroplasty of the hip under general anesthesia was admitted. Preoperative chest X-rays revealed dilation of the trachea and main bronchi, but the patient exhibited no respiratory symptoms. We diagnosed her with MKS and opted for an 8.0-mm-inner-diameter reinforced tracheal tube. We positioned the cuff in the subglottic area, inflating it while monitoring for air leakage. Throughout the surgery, adequate tidal volume was maintained.
Mounier-Kuhn syndrome (MKS), or tracheobronchomegaly, is a rare disorder characterized by abnormal dilation of the trachea and main bronchi. Although the etiology of MKS remains unclear, it is attributed to atrophy or deficiency of smooth muscle cells and elastin fibers in the bronchial trees [1]. Clinical manifestations vary widely, ranging from asymptomatic to recurrent respiratory tract infections, wheezing, dyspnea, bronchiectasis, pneumothorax, emphysema, pulmonary fibrosis, and even severe respiratory failure [1,2]. Under general anesthesia, challenges such as airway leakage or collapse during mechanical ventilation may complicate the achievement of adequate tidal volume in patients with MKS, increasing the risk of pulmonary aspiration [3]. MKS often goes undiagnosed preoperatively due to patients’ lack of symptoms or clinician oversight, leading to unanticipated risks and difficulties in airway management for anesthesiologists [2].
We present the case of a 94-year-old patient who was incidentally diagnosed with MKS from chest X-rays conducted before emergency hemiarthroplasty of the hip under general anesthesia, accompanied by a literature review.
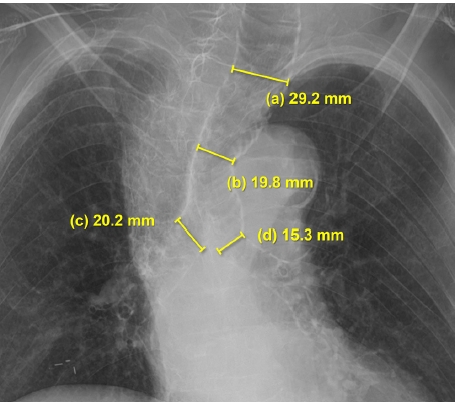
A 94-year-old woman (weight: 35 kg, height: 140 cm), usually ambulatory, was admitted for emergency hip hemiarthroplasty due to a femoral fracture sustained after slipping in her room. She had no underlying medical conditions, no previous history of surgery or anesthesia, and no family history of congenital or pulmonary disease. The patient was a nonsmoker with no significant occupational history, and she exhibited no respiratory symptoms before surgery. Her room air oxygen saturation was 99%. Echocardiography indicated an ejection fraction of 71.5%, a sclerotic and thickened aortic valve, and severe degenerative aortic stenosis. Preoperative chest X-rays showed pronounced dilation of the trachea and main bronchi, along with cardiomegaly involving a tortuous aorta and scoliosis. The trachea's longest transverse diameter measured 29.2 mm at 3.5 cm above the aortic arch, with a left main bronchus diameter of 15.3 mm and a right main bronchus diameter of 20.2 mm (Fig. 1). We diagnosed the patient with MKS.
Given the expected difficulties in airway management under general anesthesia, and considering the patient’s preoperative echocardiographic findings and the wishes of both herself and guardian, we decided to proceed with surgery under general anesthesia. By reviewing published case reports, we planned to use an endotracheal tube larger than usual and place the cuff just below the vocal cords to prevent air leakage. It was also necessary to continuously monitor the maintenance of appropriate tidal volume during surgery and to minimize any air leakage, potentially using gauze packing if required.
The patient was fasted before being brought to the operating room. Airway evaluation conducted just before anesthesia revealed no abnormalities, except that the Mallampatti classification could not be performed due to the patient’s lack of cooperation. Standard monitoring, including noninvasive blood pressure, electrocardiography, and pulse oximetry, was performed. Anesthesia was induced with 50 mg of propofol and 0.25 mg of alfentanil. Upon loss of consciousness, 35 mg of rocuronium was administered. Mask ventilation proceeded uneventfully. Intubation was performed using a conventional laryngoscope with a size 3 Macintosh blade, revealing grade I laryngeal collapse. A reinforced endotracheal tube (inner diameter [ID] of 8.0 mm, outer diameter of 11.0 mm, maximum cuff size of 28 mm) was inserted. The cuff was positioned in the subglottic area, observed through the laryngoscope, and inflated with assistance, ensuring there was no significant air leakage. The tube was secured at 17 cm from the incisors. Anesthesia was maintained using desflurane and remifentanil.
The ventilation settings (autoflow volume-controlled) were adjusted to maintain a fractional inspired oxygen (FiO2) of 40%, a tidal volume of 350 ml, and a respiratory rate of 15/min. The peak airway pressure remained at 18 mmHg, and there was no discrepancy between the set tidal volume and the actual tidal volume. Procedures for left radial artery catheterization and right internal jugular venous catheterization were successfully performed. After positioning the patient on her left side for surgery, we verified the position of the tube and checked for air leakage. Throughout the surgery, the patient’s oxygen saturation (SpO2), measured by pulse oximetry, was consistently 100%, and the end-tidal CO2 was maintained at 35-38 mmHg. Auscultation confirmed normal lung sounds on both sides, and no air leakage was detected. Arterial blood gas analysis, conducted 20 min after intubation, indicated a pH of 7.37, PaO2 of 264 mmHg, PaCO2 of 42 mmHg, and HCO3 of 24.9 mmol/L.
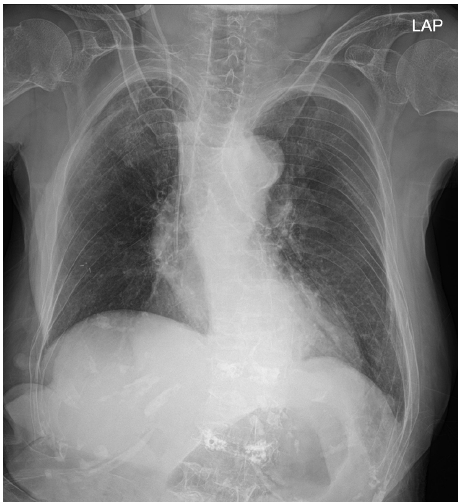
At the end of the operation, the train-of-four count was 2. Neuromuscular blockade was reversed with 100 mg of sugammadex, and extubation was carried out successfully without any complications. The total anesthesia duration was 110 min. Upon arrival in the post-anesthesia care unit, the patient received a 15-min oxygen supply at a rate of 5 L/min via a facial mask. She exhibited no respiratory symptoms, such as coughing or dyspnea. Even after the cessation of oxygen supply, the patient breathed smoothly with an SpO2 of 94%. The postoperative chest X-ray revealed pneumonia in the right upper lobe (Fig. 2), but the patient displayed no specific symptoms and required no treatment, and her recovery was uneventful. The patient and her guardian declined further evaluation of MKS. Verbal informed consent for publication of this report was obtained from the patient, who was subsequently transferred to a nursing hospital for rehabilitation on postoperative day 7 without any complications.
MKS, or congenital tracheobronchomegaly, is characterized by dilation of the trachea and main and/or proximal bronchi, with thinning of the muscular mucosa, atrophy of the longitudinal muscles, and elastic fibers being universal hallmarks of MKS [1,4]. The age range of patients with MKS varies, and its prevalence has been estimated at 0.4-1.6% in patients exhibiting pulmonary symptoms. Notably, MKS predominantly affects males, with an 8:1 male-to-female ratio [5,6]. Although patients may present with respiratory symptoms or recurrent respiratory infections, these symptoms are not specific and may vary from asymptomatic to severe respiratory failure and death [1]. MKS is often overlooked on conventional chest X-rays and is considered rare, but its actual prevalence might be higher than generally believed [5]. The etiology of MKS appears to be congenital; it is often associated with recurrent childhood infections and occasionally occurs alongside various congenital disorders. Some believe MKS to be an autosomal recessive disorder [7]. In the context of anesthesia, where effective airway management is crucial, identifying patients with MKS is essential. This case highlights the importance of thorough preoperative examinations for identifying and managing MKS patients.
If intubation in MKS patients is performed using a standard-sized tube, peritubal air leakage, inappropriate tidal volumes, and/or pulmonary aspiration may occur. Consequently, close preoperative evaluation and meticulous pre-anesthetic planning are essential [3,8,9]. The diagnosis of MKS is based on measuring the diameters of the trachea and main bronchi. These measurements can be done via chest X-ray, but chest computed tomographic scans offer more precise measurements. MKS is defined by an increase in the transverse and sagittal diameter of the trachea > 25 and 27 mm, and/or an increase in the diameter of the right and left main bronchus > 18 and 21 mm, respectively. For women, the respective measurements are 21, 23, 17.4, and 19.8 mm. An increase in the cross-sectional area of the trachea > 371 mm2 for men and 299 mm2 for women also defines the disease [1,10].
If MKS is not identified before surgery, endotracheal tube cuff-leak during intubation may be the first indication of the condition [2,5,8]. An insufficient tidal volume and reduced breathing sounds upon auscultation in both lung fields might be observed. In these situations, where cuff damage is suspected, re-intubation with a new tube might still result in an air leak, which can be perplexing. If the endotracheal tube is confirmed to be functioning correctly, an airway deformity should be considered, prompting a review of the radiograph. Solutions include using a larger-sized tube, over-inflating the cuff, repositioning the tube cuff in the subglottic area, combining oral packing with a standard bore tracheal tube, or using a laryngeal mask airway (LMA) [3,8,9,11].
No et al. [8] identified MKS in a male patient after intubation. Given the lengthy surgery duration of 8 h and the risk of tracheal wall damage due to further expansion of the cuff, they achieved an appropriate tidal volume by using a standard endotracheal tube (7.5 ID) and sealing the oropharyngeal cavity with wet gauze packing. The LMA is another option to prevent airway leakage [9]. Among these methods, the optimal approach should be selected by considering factors such as surgical duration, intraoperative posture, aspiration risk, tracheal wall damage, and airway stability.
In this case, due to the emergency nature of the surgery, the anesthesiologist reviewed only the preoperative chest X-ray directly, identifying the dilated trachea. Considering the patient’s position during surgery and the relatively short duration thereof, we used a large-size endotracheal tube with subglottic cuff inflation to establish an airtight seal, thereby minimizing the risk of airway leakage. However, employing a large-sized tube and/or excessive cuff inflation can increase the risk of mucosal injury to the trachea [3,8,9,12]. For longer surgeries, it is advisable to periodically measure cuff pressure to mitigate tracheal wall damage.
We did not perform additional cuff expansion and did not measure cuff pressure. A 28-mm cuff, positioned subglottically, was deemed appropriate to prevent both air leakage and tracheal wall damage.
While the patient did not exhibit respiratory symptoms, a careful review of the preoperative chest X-ray enabled us to identify MKS. This thorough examination facilitated the development of a comprehensive airway management plan, effectively addressing potential challenges such as airway leakage and insufficient tidal volume during general anesthesia. It is important to note that, in asymptomatic cases, MKS may go undetected until the induction of anesthesia.
In conclusion, anesthesiologists should consider the possibility of MKS in cases where preoperative radiological findings show bronchodilation and/or unexplained air leakage following tracheal intubation and cuff expansion. MKS can easily be overlooked and may lead to fatal outcomes if not appropriately managed during general anesthesia. To avoid such situations, anesthesiologists must perform a thorough evaluation of the patient, including a review of chest radiographs, and establish a suitable anesthesia plan before surgery.
Notes
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Boreum Cheon. Writing - review & editing: Ju Hyung Lee, Jae Hyung Kim, Sung Mi Hwang. Conceptualization: Sung Mi Hwang. Methodology: Sung Mi Hwang. Visualization: Ju Hyung Lee. Investigation: Jae Hyung Kim. Resources: Ju Hyung Lee. Supervision: Sung Mi Hwang. Validation: Jae Hyung Kim.
REFERENCES
1. Rjimati M, Serraj M, Elbiaze M, Benjelloun MC, Amara B. Mounier-Kuhn syndrome (Tracheobronchomegaly): radiological diagnosis. Radiol Case Rep 2021; 16: 2546-50.



2. Xiong J, Zhou Q, Li Y, Sun Y, Zhang Y. Unexpected curious cause of serious air leakage after endotracheal intubation: a case report of tracheobronchomegaly and literature review. Front Surg 2022; 9: 961186.



3. Min JJ, Lee JM, Kim JH, Hong DM, Jeon Y, Bahk JH. Anesthetic management of a patient with Mounier-Kuhn syndrome undergoing off-pump coronary artery bypass graft surgery -A case report-. Korean J Anesthesiol 2011; 61: 83-7.



4. Katz I, Levine M, Herman P. Tracheobronchiomegaly. The Mounier-Kuhn syndrome. Am J Roentgenol Radium Ther Nucl Med 1962; 88: 1084-94.

5. Krustins E, Kravale Z, Buls A. Mounier-Kuhn syndrome or congenital tracheobronchomegaly: a literature review. Respir Med 2013; 107: 1822-8.


6. Shahin S, Hoffman T, Van Es W, Grutters J, Mateyo K. Congenital tracheobronchomegaly (Mounier-Kuhn syndrome) in a 28-year-old Zambian male: a case report. Pan Afr Med J 2021; 40: 153.


7. Johnston RF, Green RA. Tracheobronchiomegaly. Report of five cases and demonstration of familial occurrence. Am Rev Respir Dis 1965; 91: 35-50.


8. No HJ, Lee JM, Won D, Kang P, Choi S. Airway management of a patient incidentally diagnosed with Mounier-Kuhn syndrome during general anesthesia. J Dent Anesth Pain Med 2019; 19: 301-6.




9. Imashuku Y, Kitagawa H, Fukushima Y, Aoi R. Anesthesia with the ProSeal Laryngeal Mask Airway for a patient with Mounier-Kuhn syndrome. J Clin Anesth 2010; 22: 154.

10. Woodring JH, Howard RS 2nd, Rehm SR. Congenital tracheobronchomegaly (Mounier-Kuhn syndrome): a report of 10 cases and review of the literature. J Thorac Imaging 1991; 6: 1-10.
-
METRICS

-
- 0 Crossref
- 259 View
- 8 Download
- Related articles in Anesth Pain Med
-
Liver transplantation of a patient with extreme thrombocytopenia - A case report -2021 July;16(3)
- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others