How does circadian rhythm affect postoperative pain after pediatric acute appendicitis surgery?
Article information
Abstract
Background
This study aimed to evaluate the relationship between postoperative pain and circadian rhythm after pediatric acute appendicitis surgery.
Methods
Two hundred patients, aged 6–18 years, undergoing acute appendicitis surgery were included in this prospective observational study. The patients were divided into four groups according to the time they underwent surgery: the night group, 01:01–07:00; morning group, 07:01–13:00; afternoon group, 13:01–19:00; and evening group, 19:01–01:00. Intraoperative and postoperative vital signs, postoperative 24-h Wong–Baker Faces Pain Rating Scale (FACEs) scores, and the amount of analgesic required were recorded.
Results
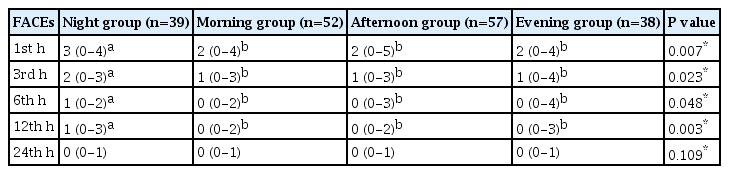
A total of 186 patients were analyzed in the study. There was no statistically significant difference in the demographic characteristics of the patient groups. Additionally, no differences were observed in intraoperative and postoperative vital signs among the four groups. However, patients in the night group had significantly higher FACEs values than those in the other groups at each time point (1st, 3rd, 6th, and 12th h) up to 12 h (P = 0.007, P = 0.023, P = 0.048, and P = 0.003, respectively). The amount of analgesic required in the night group was statistically higher than in the other groups until 12 h (P = 0.002, P < 0.001, P = 0.002, and P = 0.004, respectively).
Conclusion
A relationship was found between acute appendicitis operations performed at night (01:01 to 07:00) under general anesthesia and circadian rhythm in children. We believe that considering circadian time in the relief of postoperative pain would be beneficial.
INTRODUCTION
Any biological process that repeats itself in the body over approximately 24 h and maintains this rhythm in the absence of external stimuli is referred to as the 'circadian rhythm.' The circadian system influences numerous physiological variables, such as sleep-wake cycles, body temperature, heart rate, blood pressure, hormone secretion, metabolism, and the immune system, exhibiting self-sustaining, endogenous oscillations [1]. Clinical and experimental evidence indicates that pain sensitivity varies throughout the day in both sexes among humans and nocturnal species [2,3]. In a meta-analysis of the daily rhythms of pain sensitivity in healthy individuals, it was observed that pain sensitivity was highest at the end of the active phase and during the night [4]. However, pain threshold rhythms in humans vary significantly in response to disease, with sensitivity peaks and troughs varying inconsistently across different disease states [5]. Altered pain rhythms manifest inconsistently in various disease states. In patients with cancer, the peak of breakthrough pain attacks occurs in the late morning/afternoon [6]. Various studies have reported early morning pain peaks in patients with fibromyalgia [7] and rheumatoid arthritis [8]. In contrast, Dekkers et al. [9] did not report any diurnal changes in a rheumatoid arthritis group. Additionally, circadian differences in the efficacy of analgesia, as well as the tolerability of administration during surgical, obstetric, and dental procedures, have been observed, with most studies showing the highest pain sensitivity at night and in the early morning [10-12].
While the association between pain states and circadian rhythm has been explored in various surgical procedures and chronic pain syndromes, there is limited literature investigating the relationship between postoperative pain and circadian rhythm in pediatric surgical procedures [13]. Consequently, this study aims to assess the connection between postoperative pain and circadian rhythm following pediatric acute appendicitis surgery.
MATERIALS AND METHODS
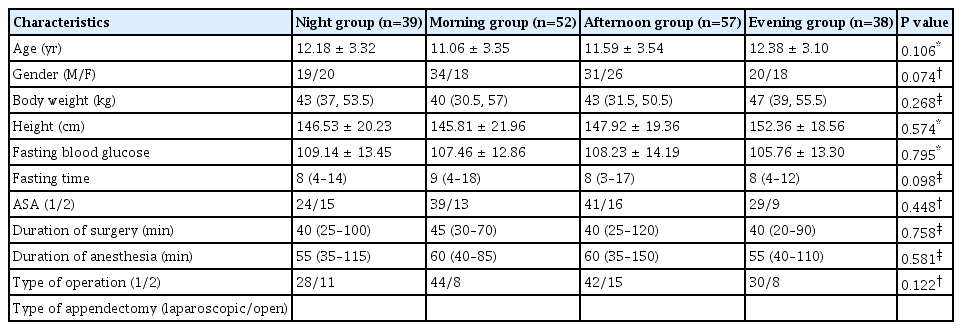
The study was prospectively planned subsequent to obtaining approval from the Selcuk University, Faculty Local Ethics Committee (IRB no. 2022/437) and Clinical Trials Registration (Ref: NCT05379192). It encompassed 200 patients, aged 6–18 years, classified with American Society of Anesthesiologists physical status I-II, who underwent acute appendicitis surgery. Exclusion criteria comprised patients meeting any of the following conditions: American Society of Anesthesiologists ≥ III, uncontrolled chronic metabolic disease, opioid use within the last 10 days, history of sepsis, abnormal surgery or recovery from anesthesia, those who underwent laparoscopic surgery, and parents unwilling to provide informed consent. Based on the operating hours, patients were categorized into four groups: night group (01:01–07:00), morning group (07:01–13:00), afternoon group (13:01–19:00), and evening group (19:01–01:00). Demographic data of the patients, including age, body weight, height, sex, American Society of Anesthesiologists scores, and anesthesia and surgery time, were systematically recorded.
Paracetamol (15 mg/kg) and midazolam (0.5 mg/kg) were administered orally as premedication to patients diagnosed with acute appendicitis, scheduled for an operation to alleviate pain until the procedure. After 3 min of preoxygenation, the standard anesthesia protocol was initiated using fentanyl (1 µg/kg) and propofol (2.5 mg/kg), followed by rocuronium (0.6 mg/kg) to facilitate anesthesia. A cuffed endotracheal tube of appropriate size was inserted into the patient’s trachea. Mechanical ventilation with oxygen-air (40–60%) and sevoflurane at 1 minimum alveolar concentration was employed, with intermittent neuromuscular blockade using rocuronium (0.2 mg/kg) if necessary. At the procedure’s conclusion, neuromuscular blockade was antagonized by intravenous injection of sugammadex (4 mg/kg), and extubation occurred upon adequate respiration and patient responsiveness to commands. Before tracheal extubation, the nasogastric tube was aspirated and removed. Intraoperative vital signs, including systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), heart rate (HR), oxygen saturation (SpO2), end-tidal carbon dioxide (ETCO2), and temperature, were recorded before surgery, at 5, 10, 15, 20, 25, and 30 min, and at the procedure’s conclusion.
Perioperative adverse effects, such as nausea, vomiting, hypotension, bradycardia, tremor, respiratory distress, sore throat, headache, and dizziness, were noted. The routine analgesic protocol, vital signs in the postoperative pediatric surgery ward, the administered amount of analgesic, the duration, and the Wong-Baker Face score (FACEs) values were also recorded. No interventions were made in the routine analgesic protocol.
A visual analog scale ranging from 0 to 10 (0 = no pain, 10 = worst pain imaginable) was utilized to assess postoperative pain intensity immediately after patients regained consciousness. A FACEs score of 2 or lower was considered acceptable for patients who experienced restful sleep. In instances of acute pain (FACEs ≥ 3), a rescue medication of 10 mg/kg intravenous ibuprofen was administered. Patients under observation in the post-anesthesia care unit were transferred to the surgical ward if their FACEs score was below 3 and the modified Aldrete score was above 8. No additional analgesics were administered until a patient requested further pain relief. During the second or subsequent request for analgesia, FACEs pain assessments were conducted. In cases where FACEs was greater than 3, an additional analgesic of 10 mg/kg (with a maximum dose of 1,200 mg/day) intravenous ibuprofen was administered at 6-h intervals.
Preliminary power analysis was performed using the 'pwr' package in R to test the difference in FACEs between the four study groups. A medium effect size (Cohen's d = 0.25) and an alpha value of 0.05 were employed for the two-tailed analysis of analysis of variance test. The results showed that a total of 180 participants, divided equally into four groups (n = 45), were required to achieve a power of 0.80. Considering a data loss rate of 10%, it was decided to include 50 patients in each group.
Statistical analysis
The statistical analyses were conducted using the R version 3.6.0 statistics package (The R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org). Normality of the data distribution was assessed using Shapiro-Wilk’s normality test and Q-Q plots. Levene’s test was employed to evaluate the homogeneity of variances. Numerical variables were reported as mean ± standard deviation, median with range (minimum-maximum), or median (interquartile range) into median (1Q, 3Q). Categorical variables were presented as counts (n). One-way ANOVA, Pearson’s Chi-square test, the Kruskal-Wallis test, and Welch’s F test were utilized to investigate potential statistically significant differences or associations between study groups based on demographic and clinical characteristics of the patients. If the variables were found to be significant with these tests, we performed multiple comparison tests (according to the assumption of normality and homogeneity of variances, Tukey's honestly significant difference post-hoc test after ANOVA, Games-Howell test after Welch's F test, and Dunn test with Bonferroni correction afterwards). A P value of less than 5% was considered statistically significant.
RESULTS
We initially assessed 200 patients for study eligibility; ten were excluded for not meeting inclusion criteria, two contracted coronavirus, and the relatives of two patients declined participation. Ultimately, 186 patients were included and analyzed: 39 in the night group, 52 in the morning group, 57 in the afternoon group, and 38 in the evening group.
No statistically significant differences were observed in the demographic characteristics of the patient groups (Table 1).
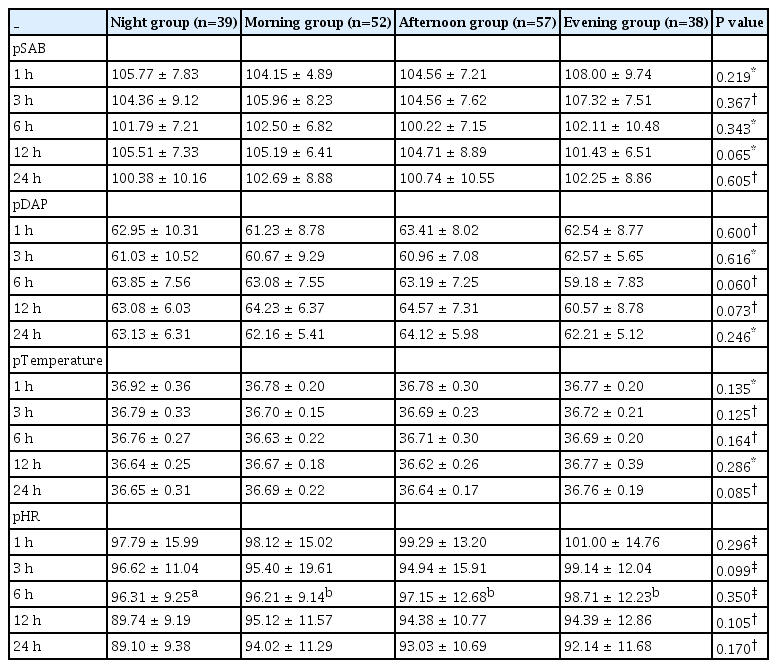
Table 2 presents preoperative and intraoperative mean values of MAP, HR, temperature, SpO2, and ETCO2 based on measurement periods. When comparing the means of SAP, DAP, and MAP across groups in terms of changes over time, no differences were found (p > 0.05) (Table 2). Similarly, there were no statistically significant differences in postoperative mean values of MAP, HR, and temperature between the groups (p > 0.05) (Table 3).
The patients in the night group exhibited significantly higher FACEs values compared to those in the other groups at each time point (1st, 3rd, 6th, and 12th h) up to 12 h (P = 0.007, P = 0.023, P = 0.048, and P = 0.003, respectively) (Table 4).
Furthermore, the amount of analgesic required in the night group was statistically higher than in the other groups for the entire 12-h period (P = 0.002, P < 0.001, P = 0.002, and P = 0.004, respectively) (Table 5). The rates of postoperative adverse effects (postoperative nausea-vomiting, bradycardia, and hypotension) were similar among the groups (P > 0.05).
DISCUSSION
Measurement of cyclical variations in pain perception can predict rhythmic changes in the analgesic requirements for individuals undergoing specific types of surgery. Pain relief can be enhanced by manipulating the timing of drug administration [3]. Our results indicate a circadian change in postoperative pain levels in children undergoing appendectomy. Patients in the night group (01:01–07:00) experienced higher FACEs than those in other circadian periods of the day. Additionally, the amount of analgesic required was higher in this group compared to the others. However, FACEs did not show any significant difference among the remaining circadian periods of the day.
While it is challenging to quantify the effects of circadian rhythms on humans in a complex clinical setting, factors such as the stress of anesthesia and surgery, administration of anesthetic drugs, and pre-operative fasting can impact the internal clock due to changes like fatigue, sleep deprivation, and inactivity, thereby lowering the pain perception threshold [14-16]. We believe that these factors contributed to the elevated pain scores observed in the night group in our study. However, our results do not seem to align with prior studies on pain processes, which demonstrate anesthesia and surgery-induced postoperative processes, associating circadian pain rhythm with higher sensitivity and a higher pain threshold during nocturnal periods [17,18]. This discrepancy may be attributed to cortisol, a potent anti-inflammatory mediator, contributing to pain reduction by decreasing endogenous proinflammatory cytokine levels [19].
In clinical practice, opioid analgesics and muscle relaxants are often administered alongside hypnotic anesthetic drugs for general anesthesia. In addition to the type of anesthesia, the administration of hypnotics, opioid analgesics, and muscle relaxants during general anesthesia in clinical practice may influence the internal clock. This is due to the critical role played by N-methyl-D-aspartate (NMDA) and γ-Aminobutyric acid (GABA) receptors in regulating the circadian clock. Furthermore, it is noteworthy that most anesthetics function as either NMDA receptor antagonists and/or GABA receptor agonists [20,21]. Auvil-Novak et al. [22] observed that, in humans, the highest and lowest demands for morphine or hydromorphone occurred in the early morning and overnight, respectively. Boom et al. [23] noted that the demand for analgesics was highest at 17:30 and lowest during the early morning hours. However, when comparing postoperative fentanyl requirements in a group of patients who underwent elective cholecystectomy at two different times, it was found that the need for fentanyl was lower in patients who had surgery early in the morning (08:00–10:00) compared to those who had surgery later (11:00–15:00) [24]. In our study, we demonstrated that postoperative pain levels in children who underwent an appendectomy were influenced by circadian rhythm. According to the circadian periods, the FACEs values of the patients in the night group (01:01-07:00) were higher in the first 12 h postoperatively compared to the other groups. Additionally, the amount of analgesic required was higher in the night group during the first 12 h postoperatively compared to the other groups.
The clinical studies conducted indicate that the pharmacokinetics of non-steroidal anti-inflammatory drugs exhibit variability throughout the day. Specifically, for ketoprofen, higher plasma peaks were observed at 07:00 [25]. In the case of indomethacin, earlier and elevated concentrations were noted when administered at 07:00 and 11:00, as opposed to other times of the day or night [26]. Controlled-release formulations of indomethacin and ketoprofen also demonstrated higher and quicker morning absorption in a separate study [27]. In a study involving healthy volunteers, the time-controlled release formulation of ibuprofen displayed lower rates and extents of bioavailability when dosed at 08:00 compared to dosing at 22:00 [28]. Conversely, immediate-release ibuprofen tablets did not exhibit any chronopharmacokinetic behavior in the same study. Moreover, an investigation involving intravenous indomethacin injected into sheep revealed that the pharmacokinetics were influenced by the animal’s biological clock [29]. However, there is currently a lack of chronobiologic data pertaining to the new cyclooxygenase-2 inhibitors.
Interestingly, some studies have shown that pain scores vary significantly throughout the day and are often specific to the type of pain experienced [30]. However, pain levels have been observed to differ across studies in relation to the circadian rhythm. Several chronic and maladaptive pain conditions are linked to changes in circadian rhythms affecting pain thresholds [31]. These altered pain rhythms manifest inconsistently across various disease states. The literature has revealed different connections between “circadian rhythm-pain” in various clinical situations [7-9,17,18,32-34]. In contrast, our results align with the circadian cycle of dental and rheumatic diseases, which exhibit peak pain levels in the early morning.
Patient conditions are difficult to maintain in clinical trials, especially when study periods extend for months, presents significant challenges. Consequently, this study has several limitations. First, the study groups were not randomly assigned but determined based on the hours of surgery, leading to an imbalance in the number of study groups. Second, most evidence regarding the regulation of pain behavior by circadian rhythms is correlative rather than causative. The mechanisms influencing circadian rhythms may vary due to differences in factors that hospitalized patients are exposed to, such as sleep/insomnia, light, noise, and meal times. Third, anxiety emerges as the most common predictor of acute postoperative pain [33]. However, measuring anxiety levels at the study's outset was not feasible. Fourth, given potential variations in postoperative pain depending on the type of appendectomy (laparoscopic vs. open), this study exclusively included open surgery cases. Furthermore, the study lacked a design or sampling methodology for measuring saliva or plasma melatonin and/or cortisol levels. Finally, the study's restriction to a single hospital may limit the generalizability of the data.
In conclusion, our findings indicate a potential relationship between acute appendicitis surgeries under general anesthesia and the circadian rhythm in children. Pediatric patients who underwent surgery between 01:01 and 07:00 experienced more pain and required increased postoperative analgesia in the first 12 h compared to patients who underwent surgery at other times of the day. Consequently, we propose that a flexible dose adjustment, accounting for circadian periods, would be highly beneficial in modern individualized pain treatment. This approach could significantly enhance patients' quality of life by minimizing the adverse effects of analgesic drugs. However, well-designed and adequately powered randomized controlled trials investigating circadian pain outcomes for both experimental and clinical studies are imperative.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Faruk Ci, cekci. Writing - review & editing: Mehmet Sargin, Fatma Ozcan Siki. Conceptualization: Faruk Ci, cekci. Data curation: Faruk Ci, cekci, Mehmet Sargin, Fatma Ozcan Siki. Formal analysis: Mehmet Sargin. Methodology: Faruk Ci, cekci. Visualization: Mehmet Sargin. Supervision: Fatma Ozcan Siki.