Neuromuscular monitoring of a patient with Charcot-Marie-Tooth disease; which monitoring technique is adequate? - A case report and literature review -
Article information
Abstract
Background
Charcot-Marie-Tooth disease (CMTD) is a hereditary polyneuropathy associated with a life-threatening risk of pulmonary complications.
Case
A 61-year-old male with CMTD for 40 years was admitted for the drainage of an abscess in his left ankle. Total intravenous anesthesia was administered, and an electromyography device was attached to the hand for neuromuscular monitoring; however, the response was not measured. Kinemyography and acceleromyography devices were attached to both hands, and responses were obtained. After neuromuscular blockade (NMB) with rocuronium 0.6 mg/kg, the train-of-four (TOF) response on kinemyography was normally measured, but the post-tetanic count on acceleromyography consistently showed 0 during anesthesia. Sugammadex 200 mg was injected to reverse the NMB. After 5 min, the TOF ratios for kinemyography and acceleromyography exceeded 90%. The patient recovered without any complications.
Conclusions
For CMTD patients, acceleromyography or kinemyography is superior to electromyography, and sugammadex can be used to reverse NMB successfully.
Charcot-Marie-Tooth disease (CMTD) is a genetic disorder characterized by progressive motor and sensory disorders [1,2]. It is the most commonly inherited neuromuscular disease, with mainly autosomal dominant (autosomal recessive or X-linked) inheritance [3]. The estimated prevalence of CMTD is 1:2,500 worldwide [4] and 1:19,230 in Korea [5]. Muscle weakness and atrophy typically develop in the distal lower limbs and slowly progress proximally, accompanied by motor and sensory deficits in all limbs over decades [2].
There are various types of CMTD: type 1 (demyelinating form) is characterized by a significant decrease in nerve conduction velocity (NCV); type 2 (axonal form) shows normal NCV with less severe clinical symptoms than those of type 1 and is a dominant intermediate type with variable NCV ranges [6,7]. The clinical manifestations are associated with various genetic anomalies and show different complications; however, distal muscle weakness and atrophy are typical clinical findings [1-3]. These clinical symptoms are mainly influenced by disease progression, specifically large axonal loss and decreased compound muscle action potential (CMAP) amplitude [7]. Despite the normal lifespan of CMTD patients, a severe clinical course can lead to life-threatening complications, such as vocal cord paralysis, pulmonary dysfunction due to respiratory muscle or diaphragm weakness, and respiratory failure [8,9].
The anesthetic management of CMTD patients should consider the possible risks of respiratory complications related to muscle weakness, autonomic dysfunction, and malignant hyperthermia [1,10,11]. Neuromuscular blockade (NMB) and its monitoring during general anesthesia are particularly challenging because CMTD is accompanied by motor weakness and peripheral polyneuropathy. After the use of neuromuscular blocking agents (NMBA), both prolonged NMB due to the loss of motor units [12-14] or denervation-like resistance [15] can occur. Therefore, adequate use of NMBA and continuous monitoring of NMB are essential in CMTD patients, and reversal of NMB to avoid postoperative pulmonary dysfunction should be considered [10,12,16,17].
This case report of abscess removal operation in a CMTD patient highlights anesthetic management from the perspective of neuromuscular transmission (NMT) monitoring, supplemented with an analysis of the literature. Written informed consent for publication of this case report was obtained from the patient after surgery. This case report is compliant.
CASE REPORT
A 61-year-old male (height, 178 cm; weight, 68 kg) visited our hospital complaining of pain and redness in the left ankle, which occurred without any specific cause. He was diagnosed with CMTD 1A in his early 20s and was not currently receiving treatment or medication. He had pes cavus foot deformity in both feet and atrophy of the peroneal muscle, which had worsened over 40 years (Fig. 1). He had a slight gait disturbance, but sensation and movement of the lower extremities remained normal. The patient’s family history revealed that his daughter also has a diagnosis of CMTD.
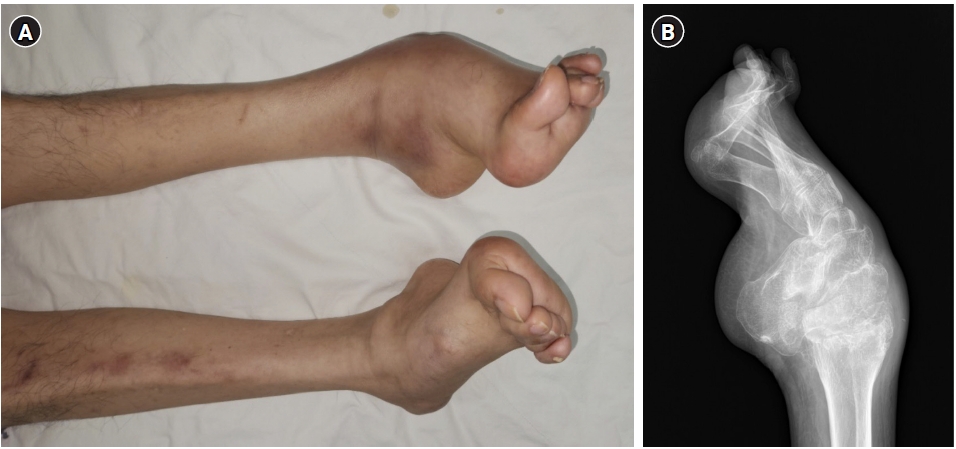
The patient exhibited weakness and atrophy of the peroneal muscle (A) with a pes cavus foot deformity (B), which are a characteristic feature of Charcot-Marie-Tooth disease.
Although we recommended regional anesthesia or peripheral nerve block with sedation, the patient insisted on general anesthesia based on his experience. He was diagnosed with an intra-articular abscess, and surgical drainage was planned. The preoperative evaluation revealed no abnormalities on chest radiography or electrocardiography. Laboratory test results were normal except for a C-reactive protein level of 33 mg/L. Before the operation, his body temperature was maintained at a slight fever above 37℃. The pulmonary function test results were within the normal range, without any respiratory symptoms. He did not complain of any other complications such as vocal cord paralysis, autonomic neuropathy, or neuropathic pain, except for diabetes, which was diagnosed approximately 10 years ago. At that time, the patient underwent lung biopsy under general anesthesia without any adverse events.
Total intravenous anesthesia with propofol and remifentanil was administered to avoid the risk of malignant hyperthermia. The patient was then transferred to the operating room without premedication. After attaching the monitoring devices, the patient was pre-oxygenated with 100% O2. An electromyography (EMG) device (EMG-NMT module of Carescape® B850, GE Healthcare) was installed on the right arm appropriately according to the guideline [18]. Anesthesia was induced with propofol (Fresofol®, Fresenius Kabi) and remifentanil (UltivaTM, Mitsubishi Tanabe) using target-controlled infusion (TCI) pumps (Perfusor Space TCI, B. Braun). The target effect-site concentration (Ce) of propofol was increased to 3.0 μg/ml, and the values of state entropy and response entropy dropped below 60.
When the patient lost consciousness, mask ventilation with 6 L/min oxygen was administered, and NMT monitoring was initiated. A schematic overview of neuromuscular monitoring and blockade in the patient is shown in Fig. 2. An automatic calibration sequence was initiated to determine the supramaximal stimulation level for NMT monitoring. However, the response to supramaximal stimulation was not detected despite the movement of the thumb, and no train-of-four (TOF) response was monitored at a stimulation current of 70 mA. The EMG device was replaced with a device that used single-use surface electrodes (TetraGraph, Senzime AB); however, the TOF response could still not be measured. Owing to polyneuropathy, EMG was deemed unsuitable; therefore, the NMT monitoring device was switched to kinemyography (KMG; Carescape® B850, GE Healthcare) on the right hand and acceleromyography (AMG; TOFscan, IDMed) on the left hand. After an automatic calibration sequence, supramaximal stimulation was performed at a current of 70 mA in the KMG. The response in AMG was measured at 60 mA. Subsequently, 2-Hz TOF monitoring of the adductor pollicis muscles was performed every 12 s.

Schematic overview of neuromuscular monitoring and blockade of the patient. EMG: electromyography, AMG: acceleromyography, KMG: kinemyography, TOF: train-of-four, PTC: post-tetanic count.
We planned to administer a small dose of rocuronium owing to the risk of delayed recovery from NMB [13], and 21 mg of rocuronium (0.3 mg/kg), a 95% effective dose, was administered. However, the TOF count (TOFc) remained at 4, and the amplitude of T4 was not reduced by more than 50% until 5 min. Thus, we administered an additional 21 mg of rocuronium, and the TOFc dropped to 0 after 2 min. The patient was then intubated, and TOF stimulations were administered every 1 min for monitoring TOFc or TOF ratio (TOFr) on the right hand using KMG. Simultaneously, the automatic post-tetanic count (PTC) mode of AMG was applied on the left hand. The TCI of propofol (Ce 2.0 to 4.0 μg/ml) and remifentanil (Ce 1.0 to 1.5 ng/ml) was used to maintain the anesthesia. The patient’s vital signs and hypnosis remained stable. His body temperature was maintained within normal limits using a forced air warmer (Bear Hugger®, 3M Company).
Thirty minutes after intubation, TOFc on KMG began to appear and reached 4 within 5 min. However, PTC on AMG consistently showed 0. Since it was difficult to objectively evaluate the degree of NMB and there was not much time left until the end of the surgery, we did not administer additional rocuronium. The surgery ended after approximately 60 min of intubation, and the TCI pump was turned off. At that time, the TOFr of the KMG was 0.34, whereas that of the AMG was 0. The AMG mode was converted to TOF, and the TOFc was 0. For the safe reversal from NMB, 150 mg (2 mg/kg) of sugammadex (Bridion®, MSD) was administered intravenously. Three min after sugammadex administration, TOFr began to appear on the AMG. After 10 min of sugammadex administration, TOFr was > 0.9 on both AMG and KMG, and self-respiration was restored. After the recovery of > 500 ml of tidal volume, the patient awakened. Extubation was carefully performed, and the patient was transferred to the recovery room. No respiratory depression or discomfort was reported in the recovery room. The next day, chest radiography and pulmonary function tests showed normal findings with forced vital capacity and forced expiratory volume in one second of 84.6% and 90.4% of the predicted normal values, respectively. The patient maintained stable hemodynamic and respiratory conditions in the ward. He was discharged without complications.
DISCUSSION
This case report presented a difficulty in obtaining reliable NMT monitoring results in a CMTD patient, which is essential for managing NMB. In this case, EMG could not detect the supramaximal stimuli or measure the TOF response, whereas KMG and AMG could be performed. These findings emphasize the need to employ appropriate NMT monitoring techniques for polyneuropathy patients such as CMTD.
Anesthetic management of CMTD patients can be challenging owing to the complex clinical characteristics of progressive motor and sensory deficits [1-3,8-11,19], as well as variable responses to NMBAs [12-15]. Choosing the correct rocuronium dose under these circumstances is a key difficulty. There is a potential for the development of prolonged postoperative NMB caused by a non-depolarizing NMBA owing to the abnormal neuromuscular junction (NMJ) physiology of CMTD patients and impairment of the phrenic nerve and diaphragm [12-14,20]; however, resistance to NMBA can be found after the use of NMBA despite muscle weakness [15]. Upregulation of acetylcholine receptors at the NMJ may be caused by denervation-like polyneuropathy, and as a consequence, a normal response or moderate resistance to NMBA can ultimately be shown [21]. As the initial dose of 0.3 mg/kg (21 mg) failed to induce adequate NMB in this case, an extra equivalent dose was necessary. These findings are consistent with those of a previous report showing a normal response to NMBA [15]. Moreover, if NMT monitoring is targeted to the chronically paralyzed muscle that shows resistance to NMB, there is a risk of administering excessive NMBA compared to the amount administered for the NMB of the respiratory muscles, which requires caution. Therefore, the effects of rocuronium in CMTD patients can be unpredictable, and adequate real-time NMT monitoring to titrate the dose of NMBAs is essential in these patients to balance the risk of inadequate muscle relaxation against prolonged paralysis.
However, monitoring NMT in CMTD patients presents unique challenges. Table 1 presents previous case reports describing neuromuscular monitoring during general anesthesia for CMTD, showing various techniques, sites, and responses in NMT monitoring, indicating inter-individual variability. Although EMG-based NMT monitoring is gaining attention because of its accurate measurement and ability to overcome the problems of AMG-based equipment such as overestimation and postural limitations [22], muscle action potentials in CMDT patients may not be measurable using EMG because of its nature. In this case, supramaximal stimulation could not be obtained with EMG, and there was no TOF response despite a maximal stimulation current of 70 mA. EMG measures the CMAP, which can provide information on the number of functional fibers [22]. Demyelination neuropathy is associated with slowing of the NCV, prolongation of distal latency, and amplitude changes due to secondary axonal loss [23]. Moreover, the stimulation current required to produce minimal CMAP in CMTD type 1 patients is more than three times higher than that in normal patients [24]. Therefore, slowing of NCV, reduced CMAP of the muscles in CMT1, and a conduction block-like effect due to a high threshold for CMAP production may impair the EMG assessment of NMT [25]. Accordingly, AMG or KMG, which measures actual muscle contraction, may be more suitable for NMT monitoring in CMTD patients with muscle weakness and atrophy.

Review of Case Reports That Describe Neuromuscular Monitoring during General Anesthesia in Charcot-Marie-Tooth Disease
Interestingly, we monitored the TOF response using the KMG at 1 min interval on the right hand, while the automatic PTC mode using the AMG was applied to the left hand. The PTC remained at 0 throughout the monitoring period, although the TOFc in the contralateral hand was 4. The response to TOF stimulation was absent, even though the AMG mode was converted from PTC to TOF. Although this discrepancy between the AMG and KMG might be caused by a difference in the progress of neuronal degeneration between the two hands [26], temporal depletion of acetylcholine release from nerve endings by PTC stimulation was suspected. During the calibration and initial administration of rocuronium, both AMG and KMG showed similar responses; however, AMG showed no response during PTC stimulation. Moreover, TOFr in the AMG was observed after the use of sugammadex. Therefore, the temporal dysfunction of NMJ associated with acetylcholine may have recovered after discontinuing PTC stimulation and administration of sugammadex. In an animal model of CMTD type 1, sustained nerve stimulation decreased acetylcholine release from nerve endings and temporarily impaired NMT [27]. Demyelinating polyneuropathy in CMTD is thought to be associated with structural and functional deficits in the NMJ. Moreover, it is possible that decreased acetylcholine release by stimulation of the PTC had an effect similar to that of increased sensitivity to NMBA at NMJ [28]; thus, no TOF response was observed until sugammadex administration. Therefore, the PTC mode may not be desirable for CMTD patients because it may cause errors in quantifying deep neuromuscular blockade.
In the present case, sugammadex was used to reverse NMB. In previous reports, sugammadex has been successfully used to reverse NMB in a CMTD patient without residual NMB [11,29]. However, prolonged respiratory paralysis has been reported despite the use of sugammadex [10]. The CMTD patient with severe restrictive pulmonary impairment showed resistance to high-dose sugammadex (17.3 mg/kg against rocuronium 0.73 mg/kg for a surgical duration of 273 min) with TOFr 0. Possible mechanisms for residual paralysis include reduced acetylcholine release from presynaptic nerve endings and synaptic dysfunction of the respiratory muscles. Nonetheless, these perplexing results seem to originate from difficulties in NMB monitoring [10,26]. Reduced acetylcholine release from presynaptic nerve endings or differences in the progression of neuropathy between the two hands may have led to unreliable NMT monitoring results [10,26]. It is assumed that the residual paralysis refractory to sugammadex was due to severe weakness of the diaphragm and respiratory muscles and not residual rocuronium in the NMJ. In the present case, the TOFr on KMG was 0.5 at the end of the surgery, and the patient recovered completely from NMB after using administration and showed normal postoperative pulmonary function. Therefore, sugammadex can provide reliable recovery from rocuronium-induced NMB in CMTD patients, and respiratory function can be restored if the patient’s neuropathy is not severe enough to induce life-threatening pulmonary dysfunction. In addition. Severe distal muscular weakness or atrophy may be accompanied by decreased NCV and CMAP, leading to the misinterpretation of NMT monitoring results [10,26]. Thus, confirmation of the applicability of the NMT device to the adductor pollicis muscle, whether the responses from both hands are similar or are supramaximal stimuli, can be obtained before using NMBAs. In addition, NMT monitoring of the alternative muscles should be considered [26,30,31]. In the case of CMT patients with distal limb neuropathy, central muscles such as the corrugator supercilia or trapezius seem to provide information on adequate intubation and surgical conditions during surgery.
In summary, this case report highlights the complexities and nuances of NMT monitoring in CMTD patients. Given the complex nature of CMTD and the associated risks of postoperative pulmonary complications, it is imperative that anesthesiologists customize the NMB strategies for each patient. Adequate NMT monitoring using AMG or KMG is advisable; however, EMG has limitations in advanced polyneuropathy. The proper use of sugammadex according to NMT monitoring facilitates the rapid and safe reversal of NMB. The utility of employing alternative muscles to monitor NMT should be considered. Future research should focus on establishing evidence-based techniques for NMT monitoring in CMTD patients.
Notes
FUNDING
The present study was supported by grants from the Clinical Medicine Research Institute at Chosun University Hospital, 2023.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Seung Un Kim, Seora Kim, Ki Tae Jung. Writing - review & editing: Ki Tae Jung. Conceptualization: Ki Tae Jung. Visualization: Ki Tae Jung.
