Chronic exposure to dexamethasone may not affect sugammadex reversal of rocuronium-induced neuromuscular blockade: an in vivo study on rats
Article information
Abstract
Background
Chronic glucocorticoid exposure is associated with resistance to nondepolarizing neuromuscular blocking agents. Therefore, we hypothesized that sugammadex-induced recovery would occur more rapidly in subjects exposed to chronic dexamethasone compared to those who were not exposed. This study evaluated the sugammadex-induced recovery profile after neuromuscular blockade (NMB) in rats exposed to chronic dexamethasone.
Methods
Sprague–Dawley rats were allocated to three groups (dexamethasone, control, and pair-fed group) for the in vivo study. The mice received daily intraperitoneal dexamethasone injections (500 μg/kg) or 0.9% saline for 15 days. To achieve complete NMB, 3.5 mg/kg rocuronium was administered on the sixteenth day. The recovery time to a train-of-four ratio ≥ 0.9 was measured to evaluate the complete recovery following the sugammadex injection.
Results
Among the groups, no significant differences were observed in the recovery time to a train-of-four ratio ≥ 0.9 following sugammadex administration (P = 0.531). The time to the second twitch of the train-of-four recovery following rocuronium administration indicated that the duration of NMB was significantly shorter in Group D than that in Groups C and P (P = 0.001).
Conclusions
Chronic exposure to dexamethasone did not shorten the recovery time of sugammadex-induced NMB reversal. However, the findings of this study indicated that no adjustments to sugammadex dosage or route of administration is required, even in patients undergoing long-term steroid treatment.
INTRODUCTION
The prevention of recurarization after using neuromuscular blocking agents (NMBAs) has been the primary concern in reducing respiratory-related adverse outcomes following anesthesia. Many anesthesiologists use sugammadex to reverse neuromuscular blockade (NMB). Sugammadex is a cyclodextrin that encapsulates lipophilic compounds [1]. It selectively binds rocuronium, which contains a steroidal nucleus. This reduces the concentration of NMBA in the neuromuscular junction, enabling rapid and effective NMB reversal, even with a profound blockade [2].
Glucocorticoids have been prescribed for diseases caused by inflammation, such as chronic obstructive pulmonary disease, allergies, rheumatoid arthritis, osteoarthritis, inflammatory bowel disease, eczema, and other allergic skin conditions [3]. Glucocorticoids are used by all medical specialties. However, Soltesz et al. [4,5] reported that chronic corticosteroid treatment shortened the duration of NMB [6] because it induced modification of acetylcholine receptor (AChR) properties, leading to reduced affinity for the receptor and rocuronium molecules. Subsequently, several studies have been conducted on this topic. In particular, since the introduction of sugammadex, many studies have been conducted on the effects of a single bolus dose of the steroid on the suggammadex-induced recovery of NMB. However, clinical studies investigating chronic dexamethasone treatment at a dose known to cause muscle atrophy are still unavailable because of ethical constraints and a lack of sufficient animal testing. Nevertheless, in vivo studies in rats treated with chronic dexamethasone have not been conducted. Therefore, we conducted an in vivo study using rats to investigate the effects of chronic dexamethasone exposure on sugammadex-induced NMB reversal. We anticipated that shallow NMB would lead to a faster recovery from the blockage since chronic dexamethasone-treated AChRs have a lower affinity for rocuronium than that of untreated receptors.
Considering the resistance to NMBA caused by chronic exposure to dexamethasone, we hypothesized that chronic exposure to dexamethasone induces resistance to NMBA, resulting in faster sugammadex-induced NMB recovery.
MATERIALS AND METHODS
Assessed outcomes
The primary outcome of this study included the recovery time to a train-of-four (TOF) ratio ≥ 0.9 (TTOFr), which is the time it takes for the TOF ratio (TOFr) to recover to 0.9 or higher after the injection of sugammadex. Secondary outcomes included the time to T1 (the first twitch of TOF), recovery (TT1), and recovery index (RI). TT1 is the time taken for the T1 height to recover 95% of the baseline T1 height following sugammadex injection. RI is the time taken for the T1 height to recover from 25% to 75% of the baseline T1 height following sugammadex injection.
Animals and group assignments
Ethical approval for this study was provided by the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences, Asan Medical Center (Seoul, Korea) on February 13, 2017 (Protocol no. 2017-13-035; Chairperson Professor Jong Yeun Park). The experiments were reviewed and performed according to the guidelines and regulations established by the Institutional Animal Care and Use Committee of Asan Institute for Life Sciences, Asan Medical Center. The committee abides by the guidelines of the Institute of Laboratory Animal Resources. Rats were obtained from Orient Bio. This animal study complied with the ARRIVE guidelines [7]. The procedures were conducted in accordance with the principles outlined in the 1975 Declaration of Helsinki (revised in 2013).
We did not validate the sample size through statistical tests, but followed previous studies that have conducted experiments with approximately 10 animals per group to achieve statistically significant results [8-11]. To allow for attrition, 36 adult male Sprague–Dawley rats (7 weeks old, weighing 213–253 g) were randomly divided into three groups (n = 12 per group). Sorting was accomplished using a random number generator in Microsoft Excel 2013 (Microsoft Corp.). Only a third party who was not involved in this experiment was aware of the group allocation at different stages of the experiment. To induce chronic dexamethasone exposure at a dose previously shown to cause muscle atrophy, the dexamethasone group (Group D) received daily intraperitoneal injections of 500 μg/kg dexamethasone disodium phosphate (Yuhan) for 15 days [6,12,13]. One milliliter of 0.9% saline was used to suspend 500 μg of dexamethasone. Thus, rats weighing 213–253 g were injected with 106.5–126.5 μg of dexamethasone suspended in 1 ml of 0.9% saline. The control group (Group C) received an equivalent volume of 0.9% saline daily for 15 days. The rats in the pair-fed group (Group P) were fed with the same amount of food daily for 15 days as those in Group D. All treatments were performed in the laboratory by a third party who was not involved in the experiments.
The amount of food consumed by Group D was weighed daily, and Group P was provided with the same amount of food as Group D. Group P was pair-fed with Group D for 15 days to evaluate whether muscle dysfunction following dexamethasone treatment was caused by the anorexia typically associated with glucocorticoid therapy. Food was available ad libitum to the rats in Groups C and D. Weight of the rats were recorded daily. Dexamethasone doses were adjusted according to the changes in body weight. Water was available ad libitum to all the groups. All the mice were bred in the laboratory animal breeding room at the Laboratory Animal Research Center, Asan Institute for Life Sciences. Under specific pathogen-free conditions, the animals were housed in individually ventilated cages (Tecniplast). The rats were raised at a constant temperature of 22°C, humidity 50 ± 10%, laboratory rodent chow, and were maintained under a regular diurnal (12-h light and 12-h dark) cycle. All the injections were administered simultaneously daily. These treatments are summarized in Fig. 1.

Flow diagram of the treatment. IPI: intraperitoneal injection, Group C: control grou, Group D: dexamethasone group, Group P: pair-fed group. Group C received the same volume of 0.9% saline daily as Group D. Group D received a daily IPI of 500 μg/kg dexamethasone suspended in 1 ml of 0.9% saline. Food and water were provided ad libitum. Group P received the same volume of 0.9% saline as Group D each day and was fed daily with the same amount of food and water as Group D.
General surgical procedures
Twenty-four hours after the last drug administration, the rats were anesthetized with an intraperitoneal injection of AlfaxanTM (Jurox Pty. Limited) at 40 mg/kg body mass. Adequate depth of anesthesia was confirmed by the absence of a withdrawal response to toe clamping [14]. When there was a withdrawal response, 10–20 mg/kg body weight of anesthetic agent was administered, if necessary. The animals underwent tracheotomies, artificial ventilation to ensure normal breathing throughout the surgery, and the jugular vein catheterization to administer medications. Body temperature was monitored using an esophageal temperature probe (Regulation to 37 ± 1°C), and a warming pad and light source were used to maintain proper body temperature. The anterior tibialis muscle was exposed, and the distal part of the tendon was tied with 3–0 black silk. It was then connected to a force-displacement transducer (Grass FT03, Grass Instrument Co.) to measure the isometric contraction of the anterior tibialis muscle at a resting tension of 2 g. The sciatic nerve was exposed and connected to bipolar platinum electrodes to evaluate neuromuscular transmission.
Assessment of neuromuscular transmission
Using a nerve stimulator (S88, Grass) and a stimulation isolation unit (SIU5, Grass), TOF stimulation (frequency, 2 Hz; duration, 0.2 ms) consisting of four supramaximal square-wave pulses was applied to the sciatic nerve via bipolar platinum electrodes every 12 s throughout the study. Muscle contraction responses were recorded and digitalized with a PowerLab acquisition system (ADInstruments) and stored in LabChart7 software (ADInstruments). In all the groups, contraction responses were stabilized for at least 10 min after the initiation of TOF stimulation. The height at T1 was measured as the baseline T1 after a 10 min stabilization period. Complete NMB was achieved by administering 3.5 mg/kg of rocuronium (EsmeronTM, MSD) [6], through a jugular vein catheter [15]. When the TOF count reached zero, a complete NMB was considered. The time from the rocuronium injection to the appearance of the second TOF twitch (TT2) was recorded. When T2 appeared, 0.5 mg/kg of sugammadex (BridionTM, MSD) was administered, and TTOFr, our primary outcome, was recorded. The RI and TT1 were recorded as secondary outcomes. Fig. 2 summarizes the overall experiment.
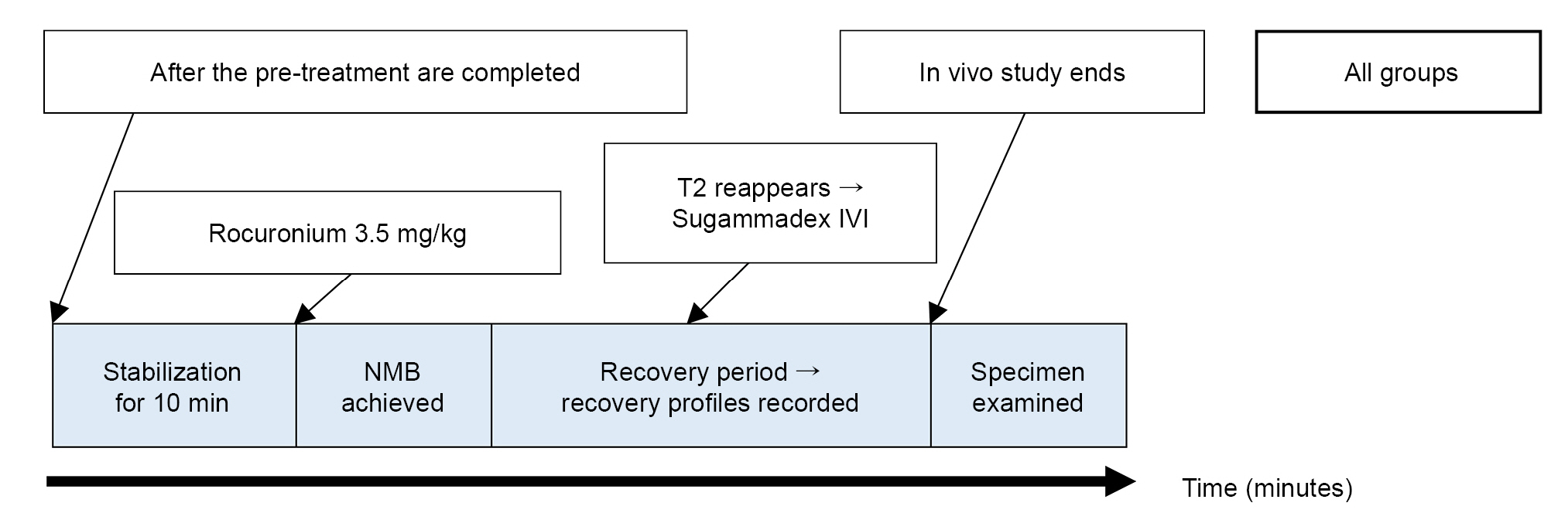
Flow diagram of the experiment. The groups included: control group (Group C), dexamethasone group (Group D), and pair-fed group (Group P). NMB: neuromuscular blockade, T2: second twitch of train-of-four stimulation, IVI: intravenous injection, and IPI: intraperitoneal injection. Group D received a daily IPI of 500 μg/kg dexamethasone suspended in 1 ml of 0.9% saline. Food and water were provided ad libitum. Group C received the same volume of 0.9% saline daily as Group D. Food and water were provided ad libitum. Group P received the same volume of 0.9% saline as Group D daily and was fed daily with the same amount of food and water as Group D.
Specimen measurement
On completion of the in vivo study, the rats were euthanized for specimen examination.
Data and statistical analysis
The primary outcome of this study was the TTOFr. The secondary outcomes were TT1 and RI. Unless otherwise specified, data were expressed as the mean ± standard deviation or median (interquartile range). Quantile–quantile plots were used to assess normality. One-way analysis of variance followed by the Tukey’s post-hoc test was applied to analyze the weight of the rats, temperature, weight of the anterior tibialis muscle, TT2, TTOFr, TT1, and RI. The Kruskal–Wallis test was used to analyze the length and width of the anterior tibialis muscle. A pairwise Wilcoxon test was applied as a post-hoc test when the Kruskal–Wallis test demonstrated a significant result.
Statistical significance was set at P values < 0.05, and all statistical tests were two-sided. SAS statistical software (version 9.3; SAS Institute Inc.) was used for statistical analysis.
RESULTS
Animal and specimen data
Thirty-six rats were allocated to three groups, with 12 rats assigned to each group. One rat in Group C was administered a rocuronium overdose, and another died during the surgical procedure. Thus, data from these two rats could not be used. We could not use data from two rats in Group D because of a ventilator breakdown resulting in respiratory failure and a computer system shutdown during the recovery process, resulting in recording errors. In Group P, data from two rats could not be used due to incomplete NMB in one rat, even after administration of the proper dosage of rocuronium with sufficient time for the onset of the drug, and another rat exhibited incomplete recovery of TOFr. Therefore, 30 rats (10 each in Groups C, D, and P) were included in the analysis (Fig. 3).
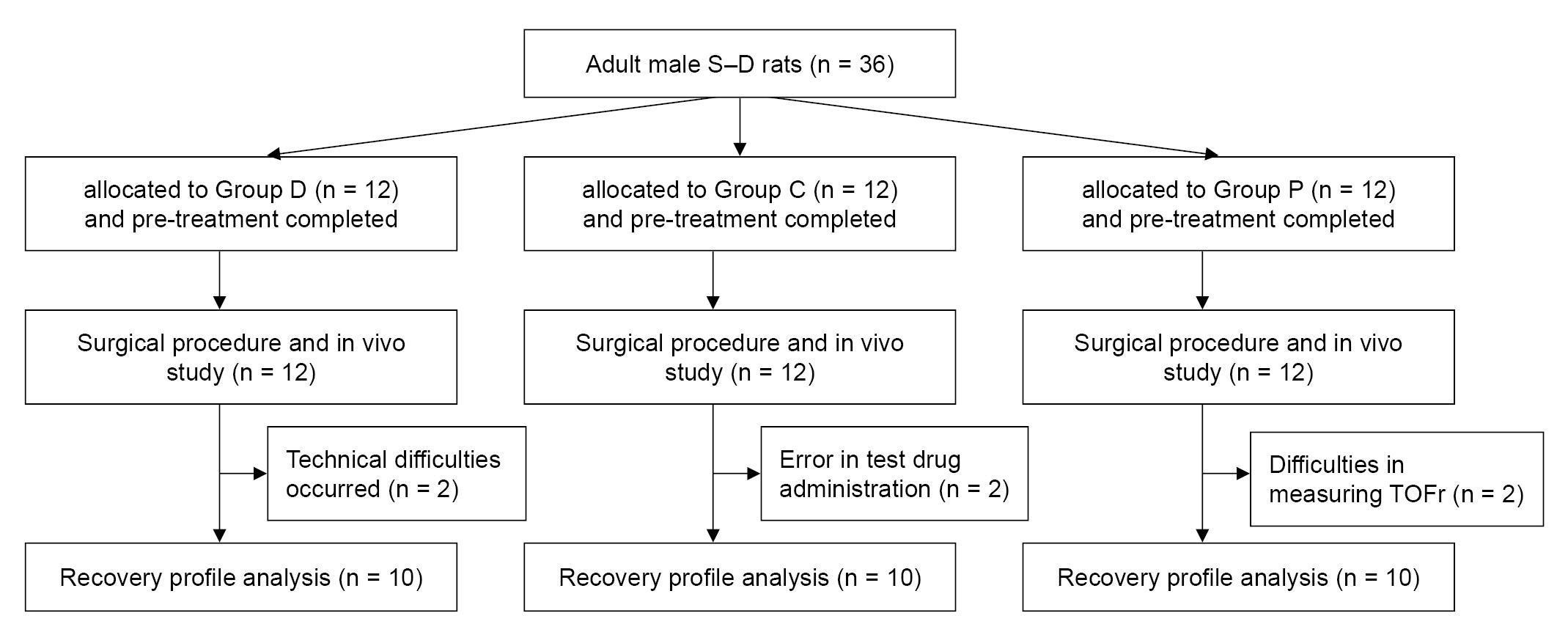
Flow chart of an experimental procedure. Group C: control group, Group D: dexamethasone group, Group P: pair-fed group, S-D: Sprague–Dawley, NMB: neuromuscular blockade, TOFr: train-of-four ratio.
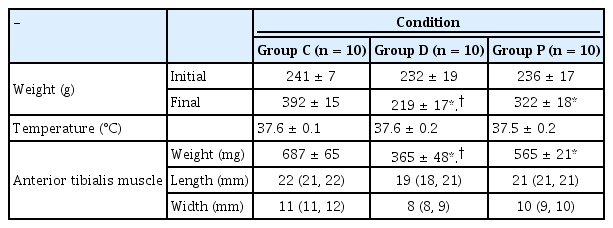
No significant differences were observed in the initial body weight before the intervention. However, the final body weight after the experimental treatment differed significantly between the groups (Table 1). As previously reported [16,17], food intake decreased markedly in Group D rats. The size and weight of the anterior tibialis muscles were smaller in Group D rats than those in Groups C and P. Though rats in Groups D and P were fed the same amount of food, the degree of weight loss, muscle size reduction, and muscle weight loss were more significant in Group P rats.
NMB induction and duration
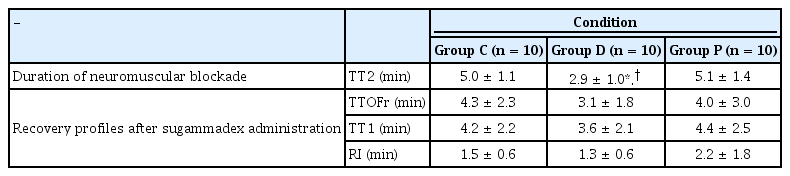
After administering 3.5 mg/kg (estimated 2-fold ED90) [18] of rocuronium via the jugular vein, a complete NMB was induced in each group. The TT2, which shows the duration of rocuronium-induced NMB, was significantly shortened in Group D rats compared to the rats in Group C and P (2.9 ± 1.0 min vs. 5.0 ± 1.1 and 5.1 ± 1.4 min; P = 0.001, respectively). Similarly, no significant difference was observed between Group C and P (P = 0.996).
Recovery profiles
The primary outcome, TTOFr, was not significantly different between the groups (4.3 ± 2.3, 3.1 ± 1.8, and 4.0 ± 3.0 min; P = 0.531 in Groups C, D, and P, respectively) (Table 2). TT1 was not shortened in Group D than in Groups C and P (3.6 ± 2.1 min vs. 4.2 ± 2.2 and 4.4 ± 2.5 min; P = 0.754, respectively) (Table 2). RI was not different between the groups (1.5 ± 0.6, 1.3 ± 0.6, and 2.2 ± 1.8 min, in Groups C, D, and P; P = 0.272, respectively) (Table 2).
DISCUSSION
In the present study, we hypothesized that the group receiving chronic dexamethasone would exhibit greater NMBA resistance and quicker sugammadex recovery. Although TTOFr and RI appeared to be shortened in Group D, no statistically significant difference was observed in TTOFr and RI between Group D and the other groups. TT1 seemed shorter in Group D, but given the wide range of its confidence interval, it was difficult to determine the significance of the results (Table 2). Therefore, we concluded that chronic dexamethasone treatment did not significantly affect sugammadex-induced NMB reversal. However, to maintain the same depth of NMB for similar duration, a more significant amount of rocuronium was required in patients receiving chronic dexamethasone treatment. Therefore, careful patient monitoring would be helpful to reduce potential adverse events.
We considered the following reasons for this observation. First, when sugammadex was administered intravenously, it bound to free NMBA molecules in the plasma, leading to differences in the concentration of NMBA molecules between the neuromuscular junctions and plasma. Consequently, NMBA molecules were released from the neuromuscular junction into the plasma and bounnd to sugammadex molecules. Therefore, regardless of the receptor affinity, the effect of sugammadex molecules continued.
Second, the reversal was extremely rapid in rats; hence, it was difficult to determine the differences in recovery profiles that might exist. The use of single-twitch stimulation would have been better. Unlike TOF stimulation, which usually occurs every 10–15 s, 1 Hz single twitch stimulation may provide a more appropriate resolution to identify fast recovery from deep neuromuscular block [18].
Concerns were raised regarding the direct binding of dexamethasone molecules to each other, which could influence NMB reversal. However, we initiated the experiment 24 h after the completion of the dexamethasone pre-treatment regimen of 14 days. Unlike the half-life of dexamethasone in humans (36–72 h), its half-life in rats is only 2.3 h [19]. Therefore, the presence of dexamethasone molecules remaining in the plasma of the experimental rats was considered negligible. Hence, it was unlikely that they would interfere with the action of sugammadex by binding to sugammadex instead of rocuronium.
It is well known that prolonged exposure to dexamethasone alters receptor characteristics. It induces nAChR upregulation [6,20,21] and the expression of the immature form of the receptor subunit [6], causing resistance to NMBA. However, this study observed that although chronic dexamethasone treatment induced resistance to NMBA, it did not affect sugammadex-induced recovery.
By comparing the results from Groups D and P, we could infer that the reduction in muscle mass caused by chronic dexamethasone exposure was not a consequence of weight loss due to reduced food intake, but rather a result of glucocorticoid-induced muscle atrophy [22]. Furthermore, by examining the recovery profiles of Groups P and C, we could deduce that although muscle mass reduction occurred, it did not affect the sugammadex-induced NMB recovery time. Therefore, reduction in muscle mass did not affect the NMB reversal time. Additionally, the duration of NMB did not change significantly in Group P. Therefore, it can be speculated that weight loss due to muscle mass reduction did not cause resistance to NMBA. However, receptor modifications due to chronic dexamethasone exposure may cause resistance to NMBA. Therefore, although Group D showed a shortened duration of NMB, the sugammadex-induced NMB recovery time remained unchanged.
Our study had some limitations. First, the sugammadex dosage used in the study was a limitation. There is no consensus on the recommended dose of sugammadex for in vivo studies in rats because of limited studies available on this topic [18,23]. Therefore, the optimal dosage of sugammadex was determined in this pilot study. The experimental dose of sugammadex was selected based on achieving a faster recovery without excessive rapid reversal from NMB. In the clinical setting, the recovery time was less than 5 min, depending on the sugammadex dose used. Using 2 mg/kg of sugammadex during moderate NMB and 4 mg/kg during deep NMB resulted in recovery times of less than 3 min and 5 min to TOFr > 0.9, respectively [24-26]. However, in our pilot study, the recovery time of TOFr was < 1 min when 2 mg/kg sugammadex was administered. Moreover, T1 recovered fully within 30 s. Unfortunately, the recovery time was so short that we could not obtain sufficient data. Therefore, we reduced the sugammadex dose to 0.5 mg/kg. This discrepancy between the in vivo study and clinical setting indicated the ongoing concern of postoperative residual neuromuscular blockade. Second, this study used only young rats (approximately 8 weeks old). If we had included rats of various age groups, similar to the clinical use of steroids in patients of different age groups, it could have provided a more accurate reflection of the clinical situation more.
In conclusion, chronic exposure to dexamethasone did not shorten the recovery time of sugammadex-induced NMB reversal. In other words, the results of this study suggested that no changes may be required in the use of sugammadex, even in patients receiving long-term steroid administration.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Ha Yeon Park, Junyong In. Data curation: Ha Yeon Park, Hey Ran Choi, Yong Beom Kim, Seok Kyeong Oh, Taehoon Kim, Hong Seuk Yang, Junyong In. Formal analysis: Ha Yeon Park, Junyong In. Methodology: Ha Yeon Park. Project administration: Ha Yeon Park, Junyong In. Visualization: Ha Yeon Park, Junyong In. Writing - original draft: Ha Yeon Park, Junyong In. Writing - review & editing: Ha Yeon Park, Hey Ran Choi, Yong Beom Kim, Seok Kyeong Oh, Taehoon Kim, Hong Seuk Yang, Junyong In. Investigation: Ha Yeon Park, Junyong In. Supervision: Junyong In.
Acknowledgements
All the authors are members of the Asan Neuromuscular Physiology Research Team at the Asan Institute of Life Science, Seoul, Korea.