 |
 |
- Search
| Anesth Pain Med > Volume 18(2); 2023 > Article |
|
Abstract
Background
The endothelial glycocalyx (EG) is an important structure that regulates vascular homeostasis. Deep inferior epigastric perforator (DIEP) flap is expected to cause substantial EG breakdown owing to the long procedural duration and ischemia-reperfusion injury. This prospective, randomized, controlled study aimed to compare syndecan-1 levels during sevoflurane-remifentanil and propofol-remifentanil anesthesia in patients who underwent DIEP flap breast reconstruction.
Methods
Fifty-one patients were randomized to either sevoflurane (n = 26) or propofol (n = 25) groups. Anesthesia was maintained with remifentanil in combination with either sevoflurane or propofol. The primary endpoint was the concentration of serum syndecan-1 measured at 1 h after surgery.
Results
Fifty patients (98.0%) completed the study. Patients in the propofol group had significantly lower levels of syndecan-1 than patients in the sevoflurane group at 1 h after operation (23.8 ± 1.6 vs. 30.9 ± 1.7 ng/ml, respectively; Bonferroni corrected P = 0.012). There were no significant differences between groups in postoperative complications. The postoperative hospital stay was 8.4 ± 2.5 days in the sevoflurane group and 7.4 ± 1.0 days in the propofol group (P = 0.077).
Conclusions
Propofol-remifentanil anesthesia resulted in lesser increases in syndecan-1 levels compared to increases with sevoflurane-remifentanil anesthesia in patients who underwent DIEP flap reconstruction. Our results suggest that propofol-remifentanil anesthesia shows protective effects against EG damage during DIEP flap breast reconstruction in contrast to sevoflurane-remifentanil anesthesia.
The endothelial glycocalyx (EG) is a gel-like layer coating the luminal surface of the vascular endothelium that functions to regulate vascular homeostasis. EG is vulnerable to degradation by various insults, including ischemia-reperfusion injury [1]. Because EG functions to regulate vascular permeability and leukocytes transmigration, damage to the EG can lead to increased vascular permeability and interstitial edema. Exaggerated inflammatory responses can also occur by upregulation of interactions between leukocytes and the endothelium [2,3]. Various surgical procedures, as well as disease states such as sepsis and major trauma, can cause degradation (also called shedding) of the EG layer, as evidenced by the increased levels of syndecan-1, a component of the core protein structure of the EG [4-6].
Deep inferior epigastric perforator (DIEP) flap reconstruction is one of the most advanced procedures for breast reconstruction following mastectomy for breast cancer [7-9]. It has gained popularity owing to its more aesthetically natural results, lower complication rates, and reduction of additional operations required [10]. However, the procedure is technically challenging and complex, and involves a lengthy duration of surgery during which meticulous adjustment of blood pressure and fluid management is required [10]. Moreover, because the flap is completely detached and blood supply is later re-established, ischemia-reperfusion injury may occur that can influence the viability of the flap [10].
Although sevoflurane anesthesia reportedly exerts some protective effects against EG degradation from ischemia-reperfusion injury in experimental studies [11,12], clinical results demonstrating a protective effect against EG of inhalation anesthetics have been inconsistent [13-15]. In one clinical study, the anesthetic agent chosen in patients undergoing minimally invasive gastrectomy has been shown to influence the degree of EG layer degradation [16]. However, to our knowledge, no studies have evaluated the influence of the anesthetic agents on the occurrence and degree of severity of glycocalyx shedding during DIEP flap breast reconstruction surgery.
Therefore, we hypothesized that sevoflurane-remifentanil and propofol-remifentanil anesthesia would have different effects on EG shedding during mastectomy with immediate DIEP flap breast reconstruction. This prospective, randomized, controlled trial aimed to compare EG shedding during sevoflurane-remifentanil and propofol-remifentanil anesthesia by comparing syndecan-1 levels in patients with breast cancer who underwent mastectomy with immediate DIEP flap breast reconstruction.
This prospective, randomized, controlled study was approved by the Institutional Review Board (IRB) and Hospital Research Ethics Committee (Yonsei University Health System, Seoul, Korea; IRB protocol no. 4-2021-0401), and registered at http://clinicaltrials.gov (ClinicalTrials.gov identifier: NCT05136508). It was conducted in accordance with the ethical principles of the Helsinki Declaration-2013, following good clinical practice guidelines. Patients aged ≥ 20 years with American Society of Anesthesiologists physical status of I to III who were scheduled to undergo total mastectomy with immediate DIEP flap reconstruction between May and November 2021 were included after obtaining informed written consent. Exclusion criteria were the need for a bilateral DIEP flap reconstruction, the inability to read or comprehend the informed consent forms, contraindications to the administration of sevoflurane or propofol, a history of thromboembolic disease, current contraceptive or thrombolytic administration, renal dysfunction (estimated glomerular filtration rate < 60 ml/min/1.73 m2), pregnancy, breast feeding, or any kind of neuropsychiatric disease.
The included patients were randomly assigned to either the sevoflurane group (n = 26) or the propofol group (n = 25) according to a randomized assignment table, which was prepared by applying the block randomization method with a block size of 4. In the sevoflurane group, anesthesia induction was initiated using a bolus dose of propofol (1.0-1.5 mg/kg) and a target-controlled infusion (TCI) of remifentanil (effect-site concentration [Ce] set to 4.0 ng/ml). In the propofol group, a commercial TIVA pump (Orchestra® Base Primea, Fresenius-Kabi) was used for both propofol and remifentanil administration, and anesthesia induction was started with a TCI of propofol (Ce of 4.0-4.5 μg/ml) and remifentanil (Ce of 4.0 ng/ml). In the sevoflurane group, anesthesia was maintained using an age-adjusted end-tidal minimal alveolar sevoflurane concentration of 0.8-1.0 and TCI of remifentanil. In the propofol group, anesthesia maintenance was performed via TCIs of propofol and remifentanil to maintain patient state index values within the range of 25-50 [17,18].
Upon entering the operating room, all patients were continuously monitored by electrocardiogram, non-invasive blood pressure, peripheral oxygen saturation, patient state index values (SedLine® electroencephalograph sensor; Masimo Corp.), and peripheral nerve stimulators. All patients received 0.1 mg of glycopyrrolate as premedication. Anesthesia was induced as described above. After confirming the patient’s loss of consciousness, a single intravenous (IV) dose of 1.2 mg/kg rocuronium was administered. Rocuronium infusion was initiated to maintain a train-of-four target of 0-2. Mechanical ventilation was set at a tidal volume of 7-8 ml per predicted body weight with 50% oxygen in air with a positive end-expiratory pressure of 5 cmH2O. Respiratory rate was adjusted to maintain the end-tidal carbon dioxide at 35-40 mmHg. Radial artery cannulation and central venous catheterization into the internal jugular vein were performed in all patients. To prevent hypothermia, a forced-air warming system was applied, and the body temperature was maintained at 36-37°C. Hypotension (mean arterial pressure [MAP] < 60 mmHg) was managed with ephedrine in 4 mg increments or with norepinephrine infusion.
For postoperative pain and nausea/vomiting, 0.08 mg/kg of IV oxycodone and 0.3 mg of IV ramosetron were administered, and postoperative neuromuscular blockade was reversed with sugammadex (Bridion®, MSD). After confirmation of spontaneous eye opening and the patient’s ability to obey commands, the endotracheal tube was removed and the patient was sent to the recovery room. During recovery, the patient was managed according to the judgment of the physician of the recovery room. After a mandatory 30 min observation period, an Aldrete score of 9 or more was required for a patient to be discharged.
The following baseline characteristics were assessed and recorded for all patients: age, sex, body mass index, American Society of Anesthesiologists physical status, comorbidities, alcohol consumption, smoking habits, and menopausal status. Tumor characteristics such as hormonal and human epidermal growth factor receptor statuses, tumor pathology, histopathologic grade, and breast cancer stage were recorded. Additionally, the following intraoperative variables were documented: duration of anesthesia, reconstruction, and total operation; the amount of anesthetic intake including the total amount of remifentanil and propofol infused; the administered doses of vasopressors including ephedrine and norepinephrine; and input and output information during the procedure such as the total fluid intake, colloid intake, blood loss, and urine output. Furthermore, the type of mastectomy and the performed lymph node procedure, the tumor size, as well as the weight of the removed breast specimen and that of the harvested flap were recorded. Finally, the duration in the recovery room, the length of the postoperative hospital stay, the required amounts of transfusion within the first 48 h after the procedure, and the patients’ postoperative adjuvant treatments were recorded. Postoperative complications including re-operations, flap detachment, hematoma, wound dehiscence, and venous congestion were analyzed.
The perioperative hemodynamic variables including MAP and heart rate (HR) were serially recorded at the following eight time points: pre-induction (baseline), 10 min after intubation (Intu10min), at the end of mastectomy (GSend), 30 min into microscopic re-anastomosis and revascularization (Micro30min), at the end of microscopic re-anastomosis and revascularization (Microend), 10 min after steep sit-up positioning (Situp10min), at the end of the operation (OPend), and 1 h after the end of operation (PO1h). Pulse pressure variation (PPV) and remifentanil Ce were recorded at seven of the above eight time points, starting from Intu10min.
The primary endpoint was the serum syndecan-1 level measured at 1 h after the end of operation. Blood samples were obtained at three time points: Intu10min, Situp10min, and PO1h. Blood samples were extracted and centrifuged at 5,000 rpm for 5 min at 4°C, after which the obtained serum was stored at a -80°C freezer until analysis. The serum analysis was performed using a specific immunoassay kit (SDC1 ELISA kit, Abnova, Cat. No. KA3851) and all samples were tested in duplicates. White blood cell count, hemoglobin levels, and neutrophil, lymphocyte and platelet count samples were collected preoperatively (Preop), at OPend, at PO1h, and on the 1st postoperative day (POD1).
The sample size was calculated based on a previous study using PASS software version 15.0.2 [16]. The calculation was performed to detect a difference of 5.9 ng/ml in the postoperative concentration of syndecan-1 between the propofol and sevoflurane groups with a significance level of 5% and a statistical power of 80%. This resulted in a total of 69 patients per group, allowing an interim analysis using Pocock’s alpha-sending function. Considering a potential dropout rate of 5%, this study was designed with 73 patients in each group. An interim analysis was performed when 25 patients had completed the study in each group. Based on the results of the interim analysis, the study was discontinued, and 50 patients were included in the final analysis.
Continuous variables are expressed as mean ± standard deviation and categorical variables are shown as the number of patients (percentage). Group differences with regards to continuous variables were determined using the student’s t-test and Chi-square test (if the portion of cells with an expected cell frequency of less than 5 was less than 20% of all the cells) or the Fisher’s exact test (otherwise) were applied for those in categorical variables. A linear mixed model analysis was employed for repeated-measure variables such as MAP, HR, PPV, remifentanil Ce, Syndecan-1 concentration, white blood cell count, hemoglobin levels, and neutrophil, lymphocyte, and platelet counts, which determined the group and time effects. When the interaction of group, time, and group-by-time showed statistical significance, post-hoc analyses with Bonferroni correction were performed to adjust for multiple comparisons. Statistical significance was defined as a P value < 0.05. All statistical analyses were performed using SAS® version 9.4 (SAS Institute Inc.).
Of the 56 patients assessed for eligibility, 51 patients were randomly allocated into the sevoflurane or the propofol group, of which 50 completed the study (98.0%). One patient in the sevoflurane group who underwent only partial mastectomy was excluded from the final analysis. A summary of the progress through the phases of the trial can be found in the Consolidated Standards of Reporting Trials flow diagram (Fig. 1).
The patients’ characteristics are demonstrated in Table 1. None of the variables were shown to have significant differences between the two groups. Table 2 presents the operative variables. Patients in the propofol group were administered with significantly higher doses of remifentanil and propofol and with significantly lower doses of norepinephrine compared to patients in the in the sevoflurane group (All P < 0.001). There were no significant differences in other operative variables between the two groups.
The perioperative changes in the MAP, HR, PPV, and remifentanil Ce are shown in Fig. 2. No statistical differences in MAP and PPV were found between the two groups (Fig. 2A and C, respectively). However, patients in the propofol group had significantly lower HRs beginning 30 min after microscopic reanastomosis until the end of operation than those of patients in the sevoflurane group (Bonferroni correct P = 0.009, 0.017, 0.034, and 0.047 at Micro30min, Microend, Situp10min, and OPend, respectively; Fig. 2B). The remifentanil Ce was significantly higher in the propofol group than in the sevoflurane group at all-time points (Bonferroni correct P = 0.016 at intu10min; Bonferroni correct P < 0.001 at all other time points; Fig. 2D).
Serum concentrations of syndecan-1 significantly increased compared with baseline values over the sampled time points in both groups. When comparing syndecan-1 levels between groups, the propofol group showed significantly lower levels of syndecan-1 compared to the sevoflurane group at Situp10min (20.1 ± 1.4 vs. 25.2 ± 1.5 ng/ml; Bonferroni corrected P = 0.049), and at PO1h (23.8 ± 1.6 vs. 30.9 ± 1.7 ng/ml; Bonferroni corrected P = 0.012). In both groups, significantly elevated serum concentration of syndecan-1 was noted at Situp10min compared to the value at baseline (Intu10min), and this increase from baseline was also seen with syndecan-1 levels at PO1h (Fig. 3).
There was no significant difference in postoperative recovery variables and complications in two groups (Table 3). Four patients in the sevoflurane group (3 cases for hematoma evacuation and 1 for venous congestion and anastomosis re-exploration) and 1 patient in the propofol group (for hematoma evacuation) underwent re-operation (P = 0.349). Postoperative hospital stay was 8.4 ± 2.5 days in the sevoflurane group and 7.4 ± 1.0 days in the propofol group (P = 0.077).
Table 4 indicates the perioperative laboratory values. Patients in the propofol group showed significantly lower WBC and neutrophil count at 1 h after operation than those in the sevoflurane group (P = 0.022 and 0.014, respectively), whereas no differences in laboratory values between groups were found at POD1. The hemoglobin levels, lymphocyte counts, and platelet counts did not differ between the groups at any of the sampled time points.
This prospective, randomized, controlled trial is the first to compare syndecan-1 levels during sevoflurane-remifentanil and propofol-remifentanil anesthesia in breast cancer patients who underwent mastectomy with DIEP flap reconstruction. With propofol-remifentanil anesthesia, the increase in syndecan-1 levels was less pronounced than that with sevoflurane-remifentanil anesthesia. Our results also demonstrate that the choice of anesthetic can have a significant effect on the shedding of syndecan-1 following DIEP flap breast reconstruction.
Similar to disease states, surgical procedures are known to cause EG shedding, and when different surgical procedures are compared, the extent of increase in syndecan-1 levels differs, presumably due to the invasiveness of the procedure [13,16,19,20]. While syndecan-1 levels increased only 20% after a minimally invasive gastric cancer surgery [16], major abdominal surgeries can result in a 40-70% increase [19]. Increases of 30-40% were observed in patients who underwent lung resection [13], while levels as high as 65 times that of the baseline value have been reported in patients who had been on cardiopulmonary bypass [20]. In the current study, syndecan-1 levels increased by an average of 68% at 1 h after operation, compared with the baseline levels. Such an increase is similar to that of increases after a major abdominal surgery [19]. Although it is a relatively superficial procedure, DIEP can cause as much of an increase in syndecan-1 levels as major abdominal surgery, presumably, due to 1) the long duration of the surgical procedures, which often lasts several hours, and 2) the occurrence of ischemia-reperfusion injury during flap harvesting and subsequent revascularization.
When the two groups were compared, in the propofol group, the percentage of increase in syndecan-1 levels was 21% and 47% at 10 min after sit-up and 1 h after operation, respectively, whereas in the sevoflurane group, the percentage of increase was 57% at 10 min after sit-up and 93% at 1 h after operation. This difference in percentage increase between groups is significant enough to be comparable to the difference in syndecan-1 levels observed among four different surgical procedures [13,16,19,20]. To reiterate, our results indicate that the choice of anesthetics for mastectomy and DIEP flap reconstruction can cause vastly different levels of increase in syndecan-1 levels.
Inhalation anesthetics exerts some protective effects against EG degradation from ischemia-reperfusion injury [11,12]; however, clinical results have been inconclusive [13-15]. The results of the present study are more consistent with those of another study in which patients who underwent minimally invasive gastrectomy exhibited lower levels of syndecan-1 in the immediate postoperative period with propofol-remifentanil anesthesia than with sevoflurane-remifentanil anesthesia [16]. It should be noted that, since both propofol and sevoflurane anesthesia are balanced anesthesia techniques utilizing opioids, the effect of remifentanil combined with either propofol or sevoflurane needs to be considered when interpreting the results. In addition, in this study, the lower white blood cell and neutrophil counts in the propofol compared to the sevoflurane group at 1 h after surgery also indicate reduced inflammation. However, definitive conclusions regarding the mechanisms by which EG degradation occurs in DIEP flap reconstruction surgery and the effect of different anesthetic agents on these mechanisms cannot be made in the present study, and further experimental studies and clinical trials are needed.
DIEP flap surgery is a complex procedure that requires meticulous management of fluid input and output, maintenance of adequate perfusion, judicious use of vasopressors, and other manipulations to avoid or minimize thrombosis [10]. EG degradation can lead to interstitial edema due to increased vascular permeability, or thrombosis due to deranged hemostasis [2,3]. Therefore, focusing on the prevention of EG shedding could be an important strategy to improve the survival rates of flap in patients following DIEP flap reconstruction surgery. We propose to choose an anesthetic that minimizes syndecan-1 shedding, whenever possible.
A study reported an incidence of free flap failure of 0.9% [21], and another study, which examined 956 flap surgeries, reported that 48 out of those cases required revision, which is equal to 5% [22]. Considering the incidence of these complications, the present study was probably unable to detect differences in the incidence of revision or postoperative complications due to insufficient number of study subjects; future studies are hence warranted.
It should further be mentioned that the administered dose of norepinephrine was significantly lower in the propofol group than in the sevoflurane group. Although poor free flap perfusion as a consequence of vasopressor use is not robustly supported by reliable prospective clinical evidence, it remains a concern for many surgeons [23,24]. While MAP and PPV were similar in both groups, significantly lower doses of norepinephrine were administered in the propofol group to maintain statistically similar hemodynamics during the procedure. This could be another advantage of propofol-remifentanil anesthesia over sevoflurane-remifentanil anesthesia for DIEP flap breast reconstruction surgery.
This study has a few limitations that need to be mentioned. First, the last measurement of syndecan-1 was performed at 1 h after the operation, and we did not obtain samples for the analysis of syndecan-1 level thereafter. Peak syndecan-1 levels are thought to occur in the 24-h period after the main insult; however, we were unable to draw any conclusions regarding those syndecan-1 trends. This shortcoming should be addressed in future studies. Secondly, although our study was sufficiently powered to detect significant differences in syndecan-1 levels, it could not detect differences in the incidences of revision rates or postoperative complications. This will also need to be addressed in a further study, focused on the incidence of postoperative complications. Third, we did not measure ischemia time of the free flap, more specifically, the time from the end of flap harvesting to start of revascularization, which is known to influence the degree of ischemia-reperfusion injury. Finally, in the present study, the remifentanil dose administered was not controlled between the two groups, being consistently higher in the propofol group than in the sevoflurane group. This increased dosage of remifentanil may affect less increased level of syndecan-1 observed in propofol group [25]. Remifentanil which is a potent µ receptor agonist is known to exert immunosuppressive effects. Moreover, there are several proposed mechanisms and sites of action including a direct action on immunocytes, and modulation of the hypothalamic-pituitary-adrenal axis, sympathetic activity, and central immunity [26,27]. However, Zongze et al. [28] showed that remifentanil had a protective effect against sepsis via both suppression of inflammatory factor production and the inducible nitric oxide synthase expression. Thus, such confounding factors including ischemic time, dosage of remifentanil, total operation, and reconstruction time other than propofol, may have influenced our results. Therefore, further controlled studies are necessary.
In conclusion, propofol-remifentanil anesthesia led to a reduced increase in syndecan-1 levels compared to sevoflurane-remifentanil anesthesia in patients with breast cancer undergoing mastectomy and DIEP flap breast reconstruction. We demonstrated that DIEP flap breast reconstruction results in a significant increase in syndecan-1 levels, suggesting that it is associated with substantial EG degradation. Furthermore, the results suggest that propofol-remifentanil anesthesia may have a beneficial effect on free flap survival compared to sevoflurane-remifentanil anesthesia when used in patients undergoing mastectomy and DIEP flap breast reconstruction. Further large-scale controlled experimental studies and clinical trials are needed.
Notes
FUNDING
This research was supported by grant No. KSA-2021 from the Korean Society of Anesthesiologists.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article but are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Bahn Lee, Na Young Kim. Data curation: Bahn Lee, Ki Hong Kweon. Formal analysis: Hye Jung Shin. Visualization: Bahn Lee, Na Young Kim. Writing - original draft: Bahn Lee, Hye Jung Shin. Writing - review & editing: Na Young Kim. Investigation: Bahn Lee. Supervision: Na Young Kim. Validation: Bahn Lee.
Fig. 2.
Perioperative changes in the (A) mean arterial pressure (MAP), (B) heart rate (HR), (C) pulse pressure variation (PPV), and (D) remifentanil effect-site concentration (Ce). Values represent the estimated means with standard error from linear mixed models. Baseline: pre-induction, Intu10 min: 10 min after intubation, GSend: mastectomy end, Microstart: microscopic reanastomosis start, Micro30min: 30 min into microscopic reanastomosis, Microend: microscopic reanastomosis end, Situp10min: 10 min after sitting position, OPend: end of operation, PO1h: 1 h after operation, Ce: effect-site concentration. *Bonferroni-corrected P < 0.050 vs. sevoflurane group.
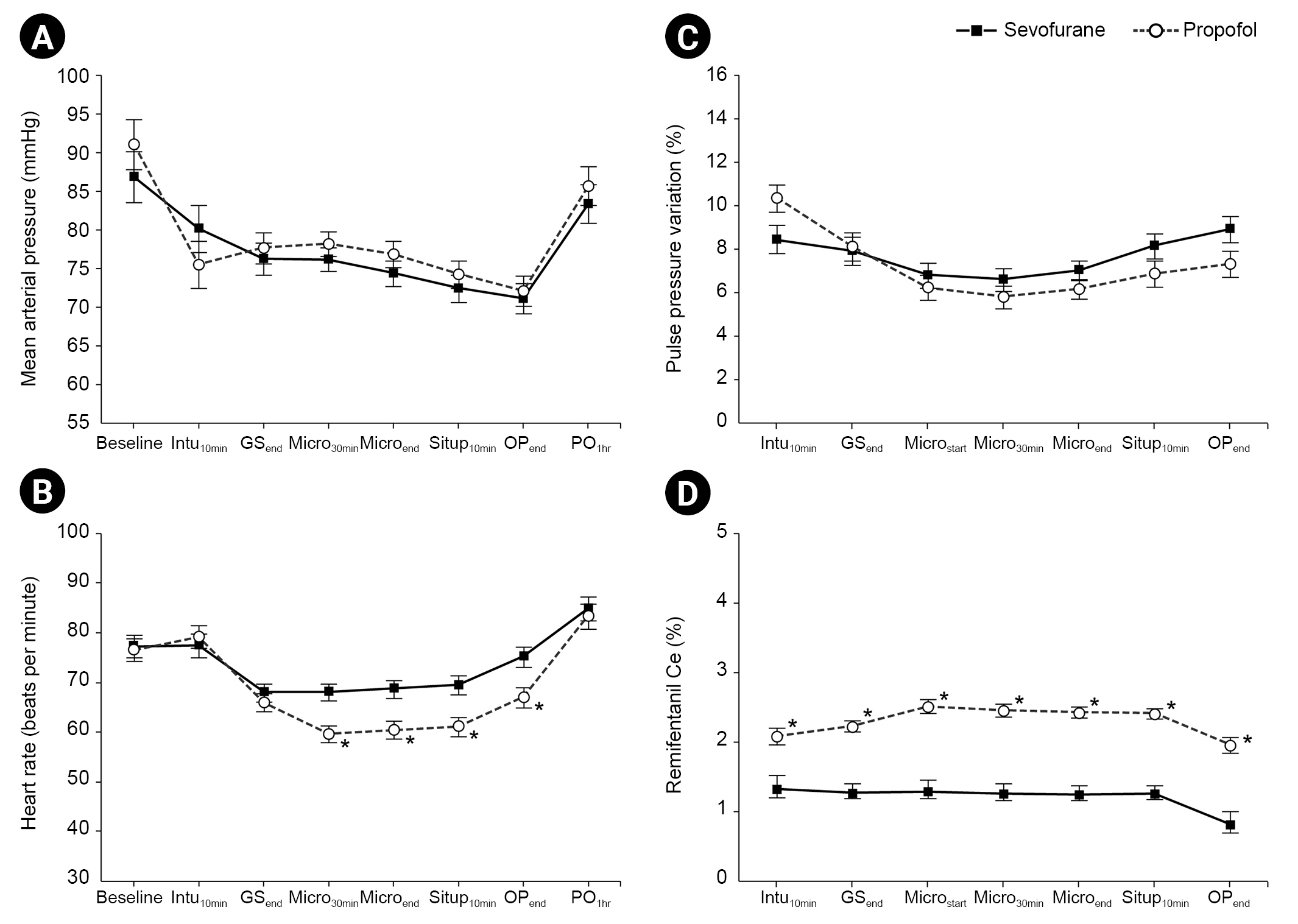
Fig. 3.
Serum concentration of syndecan-1. Values represent the estimated means with standard error from linear mixed models. Intu10 min: 10 min after intubation, Situp10min: 10 min after sitting position, PO1h: 1 h after operation. *Bonferroni-corrected P < 0.050 vs. sevoflurane group. † Bonferroni-corrected P < 0.050 vs. Intu10 min.
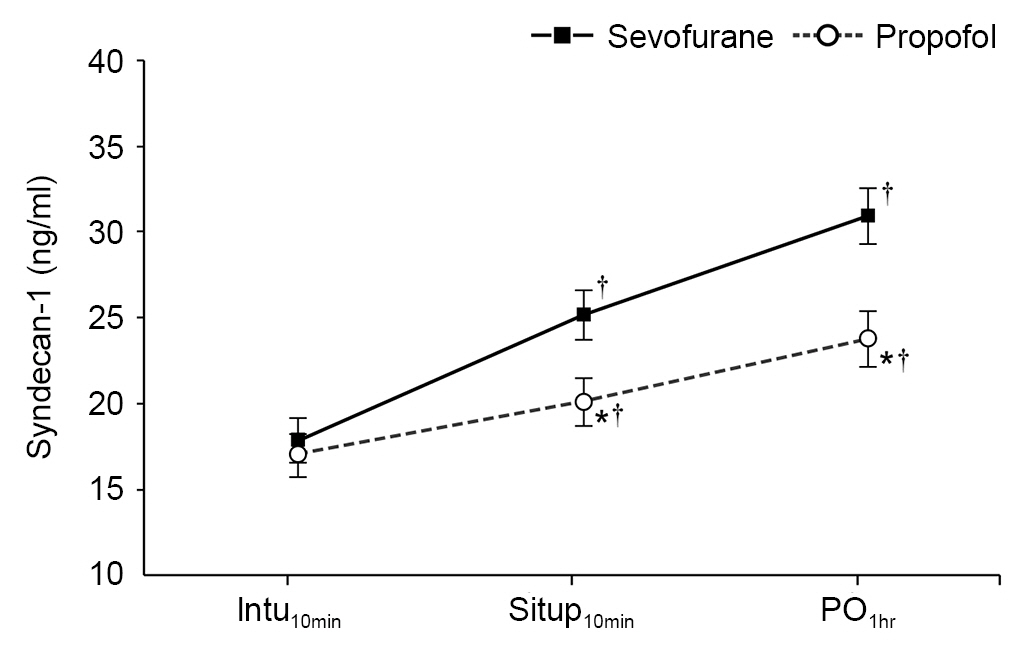
Table 1.
Patients’ Characteristics
Table 2.
Operative Variables
| Variable | Sevoflurane group (n = 25) | Propofol group (n = 25) | P value |
|---|---|---|---|
| Intraoperative variables | |||
| Duration of anesthesia (min) | 581 ± 72 | 553 ± 48 | 0.122 |
| Duration of reconstruction (min) | 399 ± 62 | 366 ± 49 | 0.055 |
| Duration of total operation (min) | 539 ± 73 | 504 ± 46 | 0.078 |
| Administered dose of remifentanil (μg) | 1,652 ± 411 | 2984 ± 594 | < 0.001* |
| Administered dose of propofol (mg) | 73 ± 14 | 3601 ± 697 | < 0.001* |
| Administered dose of ephedrine (mg) | 9.7 ± 7.3 | 9.8 ± 7.4 | 0.969 |
| Administered dose of norepinephrine (μg) | 1,592 ± 723 | 888 ± 466 | < 0.001* |
| Intraoperative fluid intake & output (ml) | |||
| Total fluid intake | 3,829 ± 828 | 3,566 ± 622 | 0.214 |
| Colloid intake | 320 ± 430 | 180 ± 284 | 0.182 |
| Urine output | 1,299 ± 547 | 1,398 ± 448 | 0.487 |
| Blood loss | 162 ± 87 | 124 ± 50 | 0.073 |
| Patients transfused during surgery | 1 (4) | 1 (4) | > 0.999 |
| Type of mastectomy | > 0.999 | ||
| Nipple sparing mastectomy | 15 (60) | 15 (60) | |
| Skin sparing mastectomy | 10 (40) | 10 (40) | |
| Lymph node procedure | 0.747 | ||
| SLNB only | 18 (72) | 19 (76) | |
| SLNB then ALND | 7 (28) | 6 (24) | |
| Specimen weight (g) | 514 ± 205 | 496 ± 220 | 0.765 |
| Flap weight (g) | 518 ± 73 | 504 ± 174 | 0.306 |
| Tumor size (cm) | 2.1 ± 1.6 | 2.7 ± 2.3 | 0.237 |
Table 3.
Postoperative Variables
Table 4.
Perioperative Laboratory Values
| Variable | Sevoflurane group (n = 25) | Propofol group (n = 25) | P value |
|---|---|---|---|
| White blood cell count (103/µl) | |||
| Preop | 6.1 ± 2.7 | 5.9 ± 2.5 | 0.828 |
| PO1h | 13.2 ± 3.7 | 11.1 ± 2.9 | 0.022 |
| POD1 | 10.2 ± 2.5 | 9.5 ± 2.7 | 0.821 |
| Hemoglobin (g/dl) | |||
| Preop | 12.6 ± 1.4 | 12.6 ± 1.8 | 0.916 |
| PO1h | 10.0 ± 1.2 | 10.3 ± 1.2 | 0.350 |
| POD1 | 9.0 ± 1.6 | 9.6 ± 1.4 | 0.220 |
| Neutrophil count | |||
| Preop | 3.8 ± 2.3 | 3.7 ± 2.2 | 0.940 |
| PO1h | 11.0 ± 3.3 | 8.9 ± 2.4 | 0.014* |
| POD1 | 8.5 ± 2.2 | 7.8 ± 2.7 | 0.912 |
| Lymphocyte count | |||
| Preop | 1.8 ± 0.6 | 1.7 ± 0.6 | 0.559 |
| PO1h | 1.4 ± 0.6 | 1.3 ± 0.7 | 0.701 |
| POD1 | 0.9 ± 0.3 | 0.9 ± 0.4 | 0.612 |
| Platelet count | |||
| Preop | 285 ± 80 | 262 ± 80 | 0.312 |
| PO1h | 205 ± 73 | 185 ± 47 | 0.250 |
| POD1 | 181 ± 54 | 182 ± 51 | 0.588 |
REFERENCES
1. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007; 454: 345-59.



2. Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 2014; 69: 777-84.


3. Kolářová H, Ambrůzová B, Svihálková Šindlerová L, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm 2014; 2014: 694312.


4. Gonzalez Rodriguez E, Ostrowski SR, Cardenas JC, Baer LA, Tomasek JS, Henriksen HH, et al. Syndecan-1: a quantitative marker for the endotheliopathy of trauma. J Am Coll Surg 2017; 225: 419-27.


5. Anand D, Ray S, Srivastava LM, Bhargava S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin Biochem 2016; 49: 768-76.


6. Suzuki A, Tomita H, Okada H. Form follows function: the endothelial glycocalyx. Transl Res 2022; 247: 158-67.


7. Gill PS, Hunt JP, Guerra AB, Dellacroce FJ, Sullivan SK, Boraski J, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg 2004; 113: 1153-60.


8. Ireton JE, Lakhiani C, Saint-Cyr M. Vascular anatomy of the deep inferior epigastric artery perforator flap: a systematic review. Plast Reconstr Surg 2014; 134: 810e-821e.

9. Selber JC, Nelson J, Fosnot J, Goldstein J, Bergey M, Sonnad SS, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: part I. unilateral reconstruction. Plast Reconstr Surg 2010; 126: 1142-53.


10. Nimalan N, Branford OA, Stocks G. Anaesthesia for free flap breast reconstruction. BJA Educ 2016; 16: 162-6.

11. Annecke T, Chappell D, Chen C, Jacob M, Welsch U, Sommerhoff CP, et al. Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br J Anaesth 2010; 104: 414-21.


12. Chappell D, Heindl B, Jacob M, Annecke T, Chen C, Rehm M, et al. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 2011; 115: 483-91.


13. Kim HJ, Kim E, Baek SH, Kim HY, Kim JY, Park J, et al. Sevoflurane did not show better protective effect on endothelial glycocalyx layer compared to propofol during lung resection surgery with one lung ventilation. J Thorac Dis 2018; 10: 1468-75.



14. Maldonado F, Morales D, Gutiérrez R, Barahona M, Cerda O, Cáceres M. Effect of sevoflurane and propofol on tourniquet-induced endothelial damage: a pilot randomized controlled trial for knee-ligament surgery. BMC Anesthesiol 2020; 20: 121.



15. Oh CS, Choi JM, Park EH, Piao L, Park HJ, Rhee KY, et al. Impact of anesthetic agents on endothelial glycocalyx injury during total knee arthroplasty: desflurane- vs. propofol-based anesthesia-a prospective randomized controlled trial. Biomed Res Int 2021; 2021: 8880267.



16. Kim NY, Kim KJ, Lee KY, Shin HJ, Cho J, Nam DJ, et al. Effect of volatile and total intravenous anesthesia on syndecan-1 shedding after minimally invasive gastrectomy: a randomized trial. Sci Rep 2021; 11: 1511.



17. Prichep LS, Gugino LD, John ER, Chabot RJ, Howard B, Merkin H, et al. The Patient State Index as an indicator of the level of hypnosis under general anaesthesia. Br J Anaesth 2004; 92: 393-9.


18. Lee KH, Kim YH, Sung YJ, Oh MK. The Patient State Index is well balanced for propofol sedation. Hippokratia 2015; 19: 235-8.


19. Steppan J, Hofer S, Funke B, Brenner T, Henrich M, Martin E, et al. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res 2011; 165: 136-41.


20. Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007; 116: 1896-906.


21. Lie KH, Barker AS, Ashton MW. A classification system for partial and complete DIEP flap necrosis based on a review of 17,096 DIEP flaps in 693 articles including analysis of 152 total flap failures. Plast Reconstr Surg 2013; 132: 1401-8.


22. Depypere B, Herregods S, Denolf J, Kerkhove LP, Mainil L, Vyncke T, et al. 20 Years of DIEAP flap breast reconstruction: a big data analysis. Sci Rep 2019; 9: 12899. Erratum in: Sci Rep 2020; 10: 1398.
23. Pattani KM, Byrne P, Boahene K, Richmon J. What makes a good flap go bad? A critical analysis of the literature of intraoperative factors related to free flap failure. Laryngoscope 2010; 120: 717-23.


24. Ibrahim AM, Kim PS, Rabie AN, Lee BT, Lin SJ. Vasopressors and reconstructive flap perfusion: a review of the literature comparing the effects of various pharmacologic agents. Ann Plast Surg 2014; 73: 245-8.

25. Zhang JN, Ma Y, Wei XY, Liu KY, Wang H, Han H, et al. Remifentanil protects against lipopolysaccharide-induced inflammation through PARP-1/NF-κB signaling pathway. Mediators Inflamm 2019; 2019: 3013716.



26. Al-Hashimi M, Scott SW, Thompson JP, Lambert DG. Opioids and immune modulation: more questions than answers. Br J Anaesth 2013; 111: 80-8.


-
METRICS

-
- 1 Crossref
- 1,834 View
- 67 Download
- Related articles in Anesth Pain Med
- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others








