Five-year all-cause mortality in critically ill liver transplant patients with coronary artery disease: analysis of acute-on chronic liver failure
Article information
Abstract
Background
Patients with acute-on-chronic liver failure (ACLF) are critically ill and have high waiting-list mortality. Although studies demonstrated that appropriately treated coronary artery disease (CAD) should not be regarded as a contraindication to liver transplant (LT), data regarding long-term outcomes in critically ill liver LT recipients are lacking. The aim of this study was to compare the rates of all-cause death at 5 years following LT in patients with ACLF with or without CAD.
Methods
Between 2010 and 2020, we evaluated 921 consecutive LT patients (MELD score, 32 ± 9) and ACLF classified by CLIF-C ACLF score. Up to 5-year all-cause death according to the CAD status was examined. CAD was defined as a preoperative history of coronary artery bypass graft or a percutaneous intervention and old myocardial infarction. Kaplan-Meier survival analysis was used.
Results
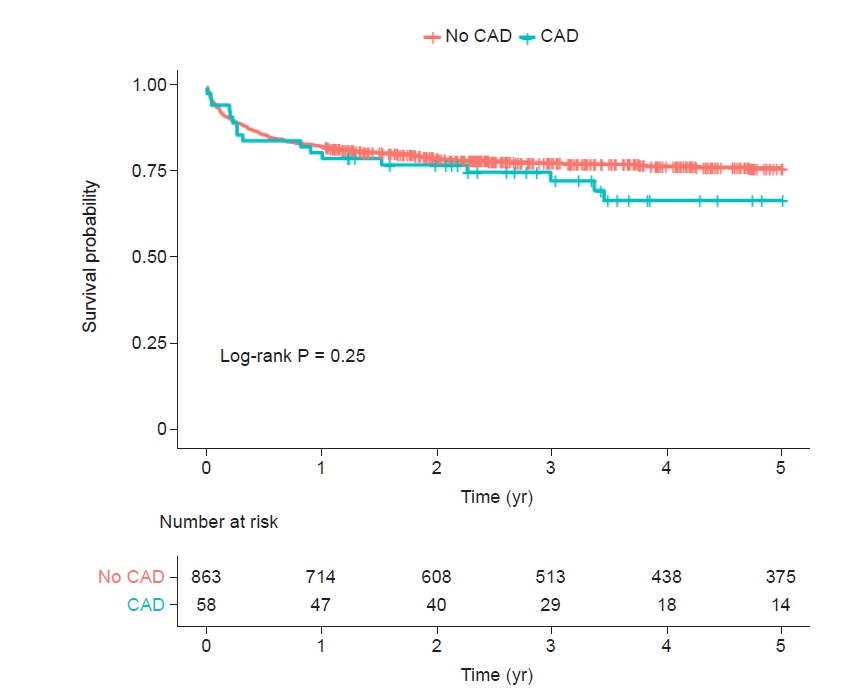
Up to 5 years, 212 (23.0%) of all ACLF patients (n = 921) in whom 17 (29.3%) of 58 CAD patients died. In patients with CAD (6.3%, 58/921), the Kaplan-Meier cumulative mortality rate at 5 years was numerically higher but was not statistically significant when compared with those without CAD (32.9% vs. 23.5%, log-rank, P = 0.25). In subgroup analysis, there were comparable risks of cumulative mortalities at 5 years across the stratification of ACLF grade 1, 2, and 3 (log-rank P = 0.062, P = 0.72, and P = 0.999, respectively).
Conclusions
All-cause mortality is high in patients with ACLF after LT but is not related to the presence of revascularized or treated CAD, across the stratification of ACLF grades.
INTRODUCTION
Cardiovascular disease (CVD) is a major contributor to short- and long-term mortality after liver transplantation (LT) in the modern era [1], ahead of graft rejection and infection, therefore it is generally considered that exact preoperative diagnosis and vigorous modification of CVD risks are mandatory [2–4].
Although severe obstructive coronary artery disease (CAD) was a contraindication at most centers previously, recent studies have demonstrated that appropriately treated or revascularized CAD should not represent a contraindication to LT [5–7].
Acute-on-chronic liver failure (ACLF), characterized by acute decompensation of cirrhosis with multi-organ failures, is associated with extremely high wait-list mortality [8]. Specifically, high-grade ACLF patients with more than three organ failures have noticeably high post-LT mortality rates [9]. Therefore, considering the escalating risk profiles of the LT candidates with ACLF, decision making for LT in critically ill ACLF patients with a previous history of CAD is clinically challenging because data to predict long-term outcomes are lacking and understudied.
The aim of this study was to compare rates of all-cause death at 5 years following LT in patients with ACLF with or without CAD, across the stratification of ACLF grade.
MATERIALS AND METHODS
Patients and data collection
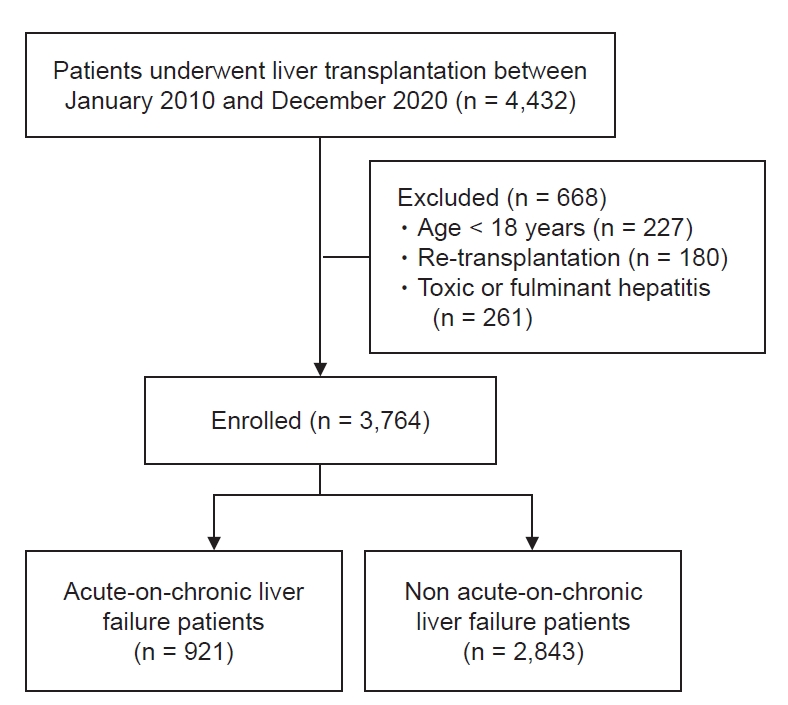
A total of 4,432 consecutive, prospectively registered patients who underwent LT from 2010 to 2020 were enrolled after approval from the Institutional Review Boards of Asan Medical Center (no. S2022-0688). Of these patients, according to the STROBE (strengthening the reporting of observational studies in epidemiology) Statement with effort to address potential sources of bias, we excluded patients with less than 18 years old, re-transplantation, toxic or fulminant hepatitis, and incomplete data (Fig. 1). Out of 3,764 end-stage liver disease who underwent LT, final 921 ACLF patients according to definition from chronic liver failure consortium ACLF score (CLIF-C ACLFs) was selected.
Patient demographics, medical history, model for end-stage liver disease score (MELDs), and laboratory and intraoperative variables were automatically obtained using a fully computerized data extraction software (ABLE). Mortality data were obtained from patients’ electronic medical records and the updated record from the institution’s LT registry.
ACLF definition and 6 organ failures
Briefly, the CLIF-C ACLFs were defined as follows; Liver failure: bilirubin level of > 12 mg/dl, kidney failure: creatinine > 2.0 mg/dl or renal replacement, brain failure: hepatic encephalopathy grade by West-Haven, 3–4, coagulation failure: international normalized ratio ≥ 2.5, circulatory failure: use of vasopressor, respiratory failure: PaO2/FiO2 ≤ 200; SpO2/FiO2 ≤ 214; or on ventilator treatment [10].
The grade of ACLF was based on the CLIF-C organ failure (CLIF-C OF) criteria including 6 failing organs (liver, kidney, brain, coagulation, circulation, and respiration). Briefly, a) presence of at least renal failure, b) any other single organ failure if associated with renal dysfunction (serum creatinine 1.5–1.9 mg/dl) and/or grade I–II hepatic encephalopathy (ACLF-grade 1). Patients with two failing organs were graded as ACLF-grade 2, while those with three or more failing organs were graded as ACLF-grade 3 [8,11].
Definition of CAD and outcome
CAD was diagnosed by cardiologists based on the pretransplant history with imaging study and angiography. CAD was considered “appropriately treated” when patients with CAD have previous history of coronary artery bypass graft or percutaneous intervention and old myocardial infarction with antianginal medications.
The primary outcome was Kaplan-Meier cumulative all-cause mortality at 5 years since the date of LT. Mortality data were collected from the medical record database and the institution’s LT registry, which is regularly updated by the Organ Transplantation Center.
Statistics
Data were expressed as mean with standard deviation or median with interquartile range (IQR) for continuous variables, and numbers and percentages for categorical variables. In univariate statistical comparisons, the chi-square test or Fisher’s exact test was used for categorical variables, Student’s t-test and Mann–Whitney test for continuous variables, as appropriate. The Kaplan–Meier survival curve was used to depict the risk of all-cause mortality up to 5 years follow-up period. The proportional hazard assumption was tested by analysis of Schoenfeld residuals and log-rank test was performed for the comparison. When the assumption was violated, Gehan–Breslow–Wilcoxon test was used. To evaluate the relationship between clinical, biochemical parameters, liver disease severity and mortality events, a Cox proportional multiple regression model was built and obtained adjusted hazard ratios (HR) for 5-year mortality. Covariates included for Cox analysis were age, sex, body mass index, diabetes, hypertension, MELD score, intraoperative red blood cell transfusion, and post-reperfusion syndrome. Statistical analyses were conducted in R (Version 4.1.2, R Foundation for Statistical Computing, Austria), with packages of ‘moonBook’ [12], ‘autoReg’ [13], “survminer” [14] “survival” [15] and a 2-sided significance level of 0.05.
RESULTS
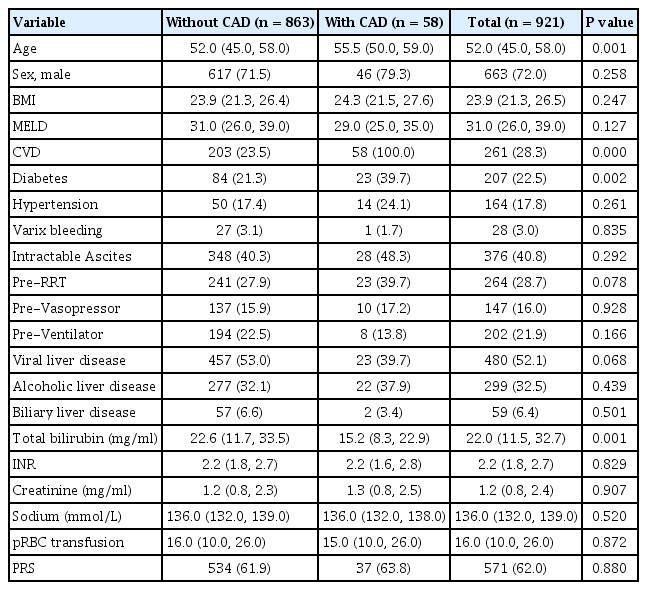
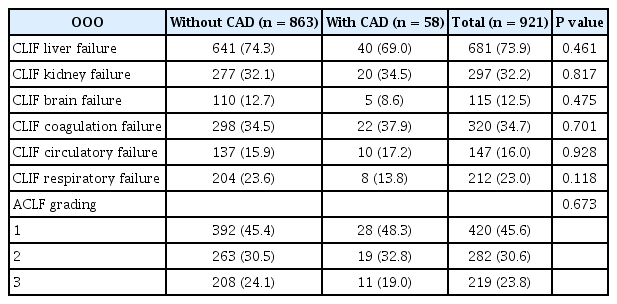
Of 921 ACLF patients included, their age was 52 (IQR: 45.0, 58.0) years and men were 663 (72%). The MELDs were 31.0 (26.0, 39.0) and total bilirubin was 22.0 (11.5, 32.7) mg/dl. The primary causes of liver disease were hepatitis B or C virus-related liver cirrhosis (52.1%), alcoholic liver disease (32.5%) and others (15.4%) (Table 1). Prevalence of organ failures defined by CLIF-C ACLFs were liver failure (73.9%), respiratory failure (23.0%), kidney failure (32.2%), coagulation failure (34.7%), circulatory failure (16.0%), and brain failure (12.5%), respectively (Table 2).
CAD patients
CAD patients (n = 58, 6.3%) showed increased age, and higher prevalence of history of previous cardiovascular disease including stroke and diabetes, but pretransplant MELD score and intraoperative transfusion amount and postreperfusion syndrome were similar (Table 1).
All-cause mortality at 5 years
In patients with CAD (6.3%, 58/921), the Kaplan-Meier cumulative mortality rate at 5 years was numerically higher but was not statistically significant when compared with those without CAD (32.9% vs. 23.5%, log-rank, P = 0.25, Fig. 2). In subgroup analysis, there were comparable risks of cumulative mortality at 5 years across the stratification of ACLF grade 1, 2, and 3 (Gehan–Breslow–Wilcoxon P = 0.19, log-rank P = 0.72, and log-rank P = 0.999, respectively, Fig. 3).
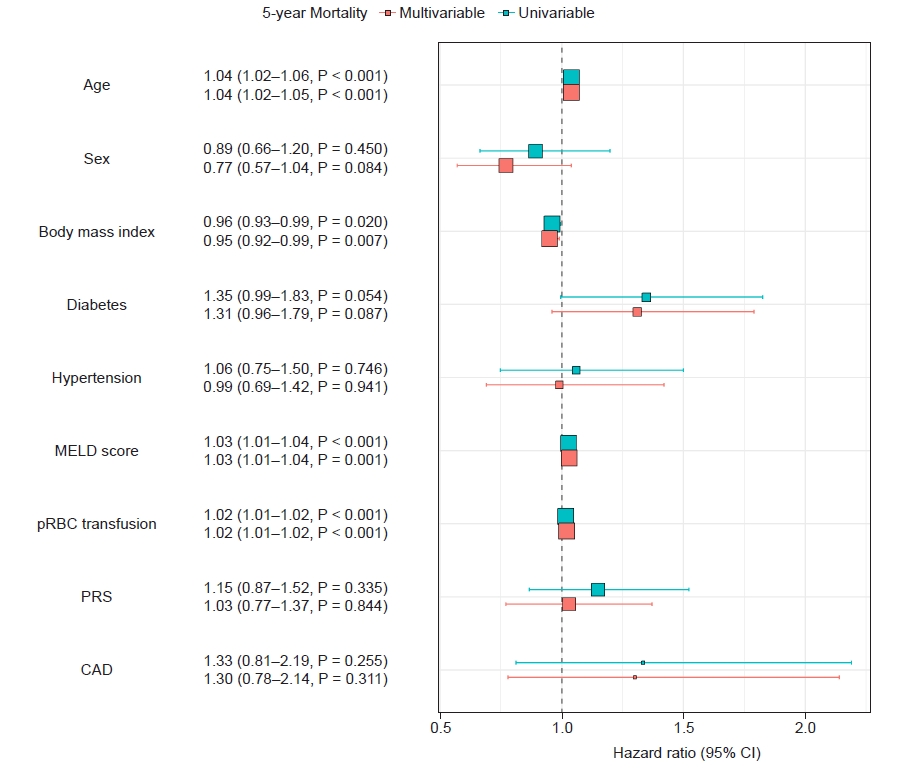
Univariable and multivariable cox-proportional hazard regression analysis for the 5-year all-cause mortality. MELD: model for end-disease liver disease, pRBC: packed red blood cell, PRS: post-reperfusion syndrome, CAD: coronary artery disease, CI: confidence interval.
In multivariable Cox proportional HR analysis, CAD did not remain as an important determinant for 5-year survival, as expected in the univariate analysis (Fig. 4).
DISCUSSION
In the current study of 921 patients with ACLF, we found that 5-year all-cause mortality is high after LT but is not related to the presence of revascularized or treated CAD, across the stratification of ACLF grade 1,2, and 3.
Of numerous ACLF risk scoring systems such as The North American Consortium for the Study of End-Stage Liver Disease's definition (NACSELD-ACLF), The Asian Pacific Association for the Study of the Liver (APASL-ACLF) [16], the established prognostic score of the European Association for the Study of the Liver-CLIF-C ACLF score has shown a greater ability to predict mortality compared to MELD incorporating sodium (MELD-Na) score in waiting list for LT [10,11,17]. Therefore, we adopted this score to grade patients with ACLF in the current study.
ACLF is associated with tremendously high short-term mortality rates when multiple organ failures develop rapidly [16,18]. Therefore, LT is believed to be the only definite treatment for patients with ACLF at present. Particularly, ACLF-grade 2 or 3 appears to benefit the most from early LT [19]. Previous studies revealed that short-term mortality of patients with ACLF grade 3 is particularly high, approaching 80% at 28-days before LT [16].
Pretransplant cardiac risk assessment can help prognosticate long-term survival, allowing adequate LT allocation and optimization of clinical outcomes [20]. Of these, CAD prevalence is increasing among LT candidates together with aging and increasing prevalence of diabetes, obesity, hypertension, and non-alcoholic fatty liver disease, therefore, asymptomatic moderate CAD is present in nearly 25% of LT candidates [21].
Moon et al. [22] demonstrated that patients with computed tomography coronary angiography (CTCA) diagnosed obstructive CAD (> 50% stenosis, 9.2% prevalence) and patients with severe stenosis (> 70% stenosis, 4.2% prevalence) showed occurrence of 6.2% and 8.9% post‐LT type 2 myocardial infarction (MI). Additionally, the prevalence of post‐LT MI increased with increasing severity of CTCA‐diagnosed CAD; the prevalence was 3.4%, 4.3%, and 21.4% in 1‐, 2‐, and 3‐vessel obstructive CAD, respectively [22].
The American Heart Association (AHA)/American College of Cardiology Foundation (ACCF) scientific statement recommends further noninvasive functional stress testing if an LT candidate has 3 or more known CAD risk factors (diabetes mellitus, hypertension, prior cardiovascular disease, left ventricular hypertrophy, age of > 60 years, smoking, and dyslipidemia) [23]. In this regard, Moon et al. [22] recommended that because the significant incidence of obstructive CAD (21.7%) in patients with 3 or more risk factors of or ≥ 3 AHA/ACCF risk factors, it seems advisable to test the CTCA in that population.
Patel et al. [7], showed that there was no evidence of a relationship between the presence and severity of CAD and composite cardiovascular events. Rather, only diabetes was associated with the likelihood of having a cardiovascular event. Therefore, they concluded that cardiovascular disease mortality is the most important contributor to early mortality after LT but is not related to the severity of CAD [7].
However, all those studies are not dedicated for the patients with critically ill LT recipients. The strength of our study is that we assessed the ACLF cohort, across the stratification of ACLF grade 1, 2, and 3. Although patients with ACLF grade 1 closely reached the statically significant P value (P = 0.062), rather more severe ill patients of ACLF grade 2 and 3 patients showed comparable results between patients with CAD and without CAD. These findings emphasize the importance of pretransplant adequate treatment of severe obstructive CAD.
Our study has several limitations, first, prospective multicenter study is recommended because of our study’s retrospective characteristics. Secondly, we did not include the nonobstructive CAD or untreated patients with CAD in this study protocol, therefore further study is needed including such patients. Thirdly, sample size of patients with CAD (n = 58) might not be sufficient for the statistical power, therefore, further study will be needed. However, this is one of the frontier studies to report the impact of CAD on post-transplant outcomes on ACLF patients.
In conclusion, 5-year all-cause mortality is not related to the presence of revascularized or treated CAD in patients with ACLF, across the stratification of ACLF grade 1, 2, and 3. As such, CAD, if appropriately treated, should not be an exclusion criterion for undergoing LT in patients with ACLF.
Notes
FUNDING
This research was supported partly by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, which is funded by the Ministry of Health & Welfare of the Republic of Korea (grant number. HI18C2383).
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Gyu-Sam Hwang. Data curation: Hye-Mee Kwon, Jae Hwan Kim, Ji-Young Kim. Formal analysis: Gyu-Sam Hwang. Methodology: Jae Hwan Kim. Writing - original draft: Hye-Mee Kwon. Software: Hye-Mee Kwon. Supervision: Gyu-Sam Hwang.