 |
 |
- Search
| Anesth Pain Med > Volume 17(4); 2022 > Article |
|
Abstract
Background
Methods
Results
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1.
Notes
FUNDING
This research was supported by the Soonchunhyang University Research Fund (2017R1C1B5076787). And these institution was not involved in any part of the study.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Sun Young Park, Wan Mo Koo. Data curation: Suyeon Park. Formal analysis: Suyeon Park. Methodology: Sokyung Yoon, Jae Hwa Yoo. Visualization: Ho Bum Cho. Writing - original draft: Sanghoon Song, Mun Gyu Kim. Writing - review & editing: Sanghoon Song. Investigation: Sang Jin Choi. Supervision: Ji Won Chung, Sang Ho Kim.
Fig. 2.
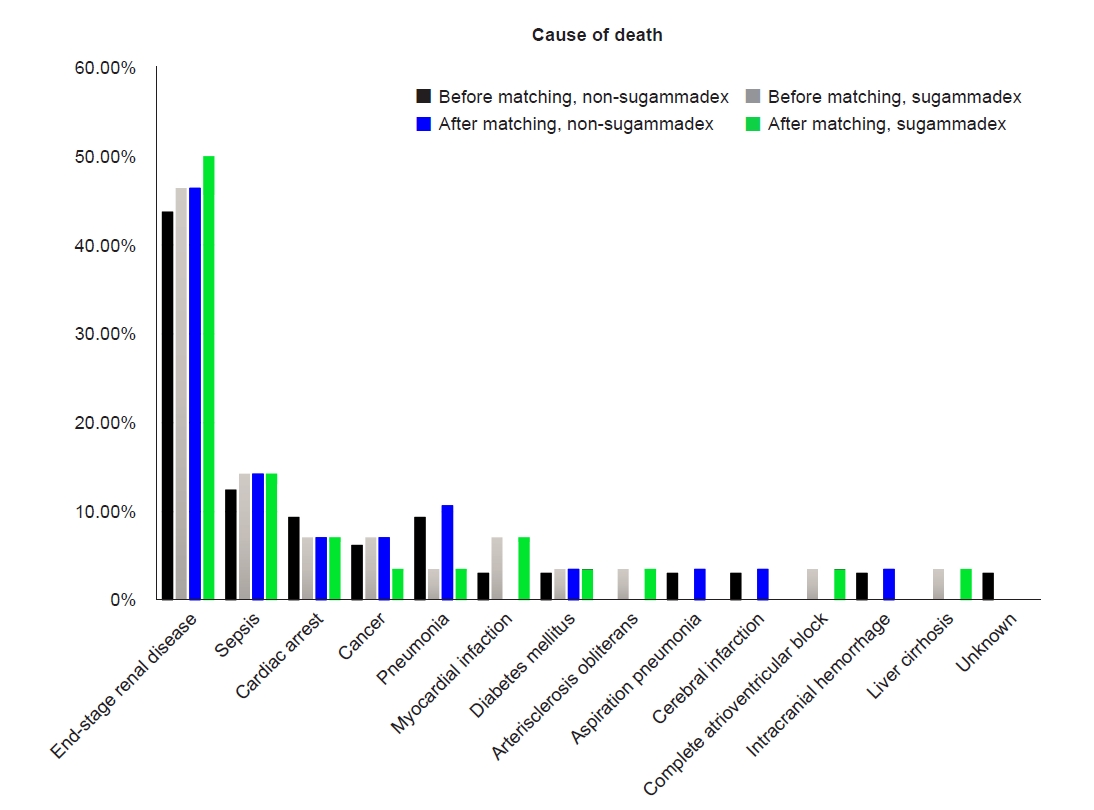
Table 1.
| Variables |
Before propensity score matching (n = 2,039) |
After propensity score matching (n = 1,594) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Sugammadex (n = 806) | Non-sugammadex (n = 1,233) | P value | SMD | Sugammadex (n = 797) | Non-sugammadex (n = 797) | P value | SMD | ||
| Age (yr) | 66 (55, 75) | 64 (55, 74) | 0.018† | 0.088 | 66 (55, 75) | 66 (57, 75) | 0.448 | 0.050 | |
| Sex, male | 444 (55.1) | 613 (49.7) | 0.020† | 0.113‡ | 440 (55.2) | 451 (56.6) | 0.614 | 0.028 | |
| Body mass index (kg/m2) | 23.1 (20.5, 26) | 22.7 (20.4, 25.6) | 0.065 | 23.1 (20.5, 25.9) | 22.8 (20.6, 25.7) | 0.318 | |||
| Hypertension | 666 (82.6) | 1005 (81.5) | 0.559 | 0.032 | 659 (82.7) | 661 (82.9) | 0.947 | 0.007 | |
| Atrial fibrillation | 57 (7.1) | 67 (5.4) | 0.156 | 0.065 | 56 (7.0) | 52 (6.5) | 0.765 | 0.020 | |
| Current angina | 62 (7.7) | 54 (4.4) | 0.002† | 0.138‡ | 61 (7.7) | 52 (6.5) | 0.435 | 0.044 | |
| Previous MI | 34 (4.2) | 37 (3.0) | 0.179 | 0.067 | 34 (4.3) | 32 (4.0) | 0.900 | 0.013 | |
| Previous congestive heart failure | 29 (3.6) | 25 (2.0) | 0.044† | 0.090 | 28 (3.5) | 22 (2.8) | 0.472 | 0.043 | |
| Valvular heart disease | 113 (14.0) | 135 (11.0) | 0.045† | 0.097 | 113 (14.2) | 118 (14.8) | 0.776 | 0.018 | |
| Dilated cardiomyopathy | 1 (0.1) | 1 (0.8) | > 0.99* | 0.014 | 1 (0.1) | 1 (0.1) | > 0.99* | < 0.001 | |
| Left ventricle ejection fraction (%) | 63 (57, 59) | 64 (60, 69) | 0.023† | 63 (57, 69) | 64 (59, 69) | 0.360 | |||
| (n = 498) | (n = 734) | (n = 492) | (n = 495) | ||||||
| Previous cerebrovascular attack | 134 (16.6) | 177 (14.4) | 0.183 | 0.063 | 133 (16.7) | 130 (16.3) | 0.893 | 0.010 | |
| COPD | 11 (1.4) | 9 (0.7) | 0.233 | 0.063 | 11 (1.4) | 9 (1.1) | 0.822 | 0.035 | |
| Diabetes mellitus | 412 (51.1) | 595 (48.3) | 0.223 | 0.065 | 410 (51.4) | 424 (53.2) | 0.514 | 0.035 | |
| Hemoglobin (g/dl) | 11.2 (10.1, 12.3) | 11.2 (10.2, 12.2) | 0.432 | 11.2 (10.1, 12.3) | 11.2 (10.2, 12.1) | 0.970 | |||
| Platelet (103/μl) | 187 (144, 229) | 182 (144, 227) | 0.373 | 186 (143, 229) | 181 (141, 226) | 0.268 | |||
| International normalized ratio | 1.00 (0.94, 1.05) | 1.01 (0.95, 1.06) | 0.277 | 1.00 (0.94, 1.05) | 1.01 (0.95, 1.07) | 0.054 | |||
| Activated partial thromboplastin time (s) | 30.8 (28.2, 33.1) | 30.6 (28.2, 33.1) | 0.847 | 30.8 (28.2, 33.1) | 30.6 (28.1, 33.1) | 0.729 | |||
| Blood urea nitrogen (mg/dl) | 29.3 (16.6, 48.8) | 27.8 (17.4, 47.23) | 0.448 | 28.5 (17.5, 47.92) | 26.3 (20.4, 38.17) | 0.363 | |||
| Creatinine (mg/dl) | 4.58 (2.37, 7.54) | 4.89 (3.14, 7.26) | 0.313 | 4.75 (2.49, 7.71) | 4.95 (3.21, 7.41) | 0.212 | |||
| Plasma Na (mmol/L) | 140 (139, 142) | 140 (138, 142) | 0.669 | 140 (139, 142) | 141 (139, 142) | 0.393 | |||
| Plasma K (mmol/L) | 4.3 (3.9, 4.8) | 4.5 (4, 4.9) | < 0.001† | 0.185‡ | 4.3 (3.9, 4.8) | 4.3 (3.9, 4.8) | 0.802 | 0.014 | |
| Plasma Cl (mmol/L) | 101 (98, 103) | 100 (98, 103) | 0.049† | 0.104‡ | 101 (98, 103) | 101 (98, 103) | 0.910 | 0.005 | |
| Plasma albumin (g/dl) | 4.2 (3.7, 4.5) | 4.3 (3.9, 4.6) | 0.001† | 0.151‡ | 4.1 (3.68, 4.4) | 4.0 (3.6, 4.4) | 0.482 | 0.091 | |
| Plasma P (mg/dl) | 4.0 (2.8, 5.1) | 4.2 (3.2, 5.3) | 0.023† | 0.172‡ | 3.8 (2.7, 4.9) | 3.8 (2.77, 4.9) | 0.954 | 0.035 | |
| Plasma Ca (mg/dl) | 9.2 (8.7, 9.7) | 9.3 (8.8, 9.8) | 0.147 | 9.2 (8.7, 9.7) | 9.2 (8.8, 9.7) | 0.822 | |||
| Hemoglobin A1c (%) | 6.6 (5.9, 7.6) (n = 380) | 6.6 (5.8, 7.6) (n = 538) | 0.858 | 6.6 (5.9, 7.6) (n = 376) | 6.6 (5.8, 7.6) (n = 392) | 0.830 | |||
| AST (U/L) | 18 (15, 23) | 17 (14, 22) | 0.156 | 18 (15, 23) | 18 (14, 23) | 0.490 | |||
| ALT (U/L) | 13 (9, 19) | 13 (10, 18) | > 0.99 | 13 (10, 19) | 13 (10, 19) | 0.589 | |||
| Intraoperative crystalloids (ml) | 150 (100, 300) | 120 (100, 200) | < 0.001† | 0.341‡ | 150 (100, 300) | 150 (100, 200) | < 0.001† | 0.269‡ | |
| Intraoperative colloids (ml) | 300 (200, 500) (n = 117) | 250 (150, 400) (n = 61) | 0.246 | 300 (200, 500) (n = 114) | 250 (200, 400) (n = 59) | 0.296 | |||
| Intraoperative blood loss (ml) | 10 (0, 40) | 20 (0, 50) | 0.065 | 10 (0, 40) | 20 (0, 50) | 0.140 | |||
| Anesthetic duration (min) | 115 (85, 155) | 110 (85, 144) | 0.002† | 0.241‡ | 115 (85, 155) | 110 (85, 140) | 0.010† | 0.221‡ | |
| Intraoperative transfusion | 12 (1.5) | 6 (0.5) | 0.032† | 12 (1.5) | 4 (0.5) | 0.076 | 0.101‡ | ||
| RBC unit of transfusion | 2 (1.75, 3.25) | 1 (1, 1.75) | 0.167 | 2 (1.75, 3.25) | 1 (1, 1.75) | 0.253 | |||
Values are presented as median (1Q, 3Q) or number (%). Because preoperative echocardiography and assessment of hemoglobin A1c were not performed routinely, and colloids were not administered routinely, the number of patients with available data for these parameters is indicated. SMD: standardized mean difference, COPD: chronic obstructive pulmonary disease, MI: myocardial infarction, Na: sodium, K: potassium, Cl: chloride, Ca: calcium, P: phosphorus, AST: aspartate aminotransferase, ALT: alanine aminotransferase, RBC: red blood cells. Continuous variables were analyzed using the Mann-Whitney U test, and categorical variables using the χ2-test or
Table 2.
Table 3.
REFERENCES
- TOOLS
-
METRICS

-
- 1 Crossref
- 3,455 View
- 97 Download
- Related articles in Anesth Pain Med
-
Cause of postoperative mortality in patients with end-stage renal disease2022 April;17(2)
- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others








