Analgesic effects of ultrasound-guided fourquadrant transversus abdominis plane in patients with cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a prospective, randomized, controlled study
Article information
Abstract
Background
Postoperative pain occurring after cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is difficult to control because of extensive surgical injuries and long incisions. We assessed whether the addition of a four-quadrant transabdominal plane (4Q-TAP) block could help in analgesic control.
Methods
Seventy-two patients scheduled to undergo elective CRS with HIPEC and intravenous patient-controlled analgesia (IV PCA) were enrolled. The patients received 4Q-TAP blocks in a 10 ml mixture of 2% lidocaine and 0.75% ropivacaine per site (4Q-TAP group, n = 36) or normal saline (control group, n = 33). Oxycodone in the post-anesthesia care unit (PACU) and pethidine or tramadol in the ward were used as rescue analgesics. The primary outcome was less than 3 times of rescue analgesic administration (%) in the ward for 5 postoperative days. Secondary endpoints included oxycodone requirement in PACU, fentanyl doses of IV PCA, morphine milligram equivalent (MME) of total opioid use, hospital stay, and postoperative complications.
Results
During 5 postoperative days, there was no difference in pain scores and total rescue analgesic administration between two groups. However, the use of oxycodone in PACU (P = 0.011), fentanyl requirement in IV PCA (P = 0.029), and MME/kg of total opioid use (median, 2.35 vs. 3.21 mg/kg, P = 0.009) were significantly smaller in the 4Q-TAP group. Hospital stay and incidence of postoperative morbidity were similar in both groups.
Conclusions
The 4Q-TAP block enhanced multimodal analgesia and decreased opioid requirements in patients with CRS with HIPEC, but did not change postoperative recovery outcomes.
INTRODUCTION
Thoracic epidural analgesia has been recognized by various guidelines as the treatment of choice among analgesic methods after open abdominal surgery due to a significant improvement in pain control, less opioid consumption, and enhancement of clinical outcomes [1,2]. However, there are still risks of infection, epidural hematoma, and failure of epidural analgesia [3].
Multimodal intravenous patient-controlled analgesia (IV PCA) contributes to enhanced recovery after surgery (ERAS) by reducing the total amount of opioids used and mixing various drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, ketamine, lidocaine [4], dexmedetomidine [5], or nefopam [6] into the IV PCA instead. With the increasing use of minimally invasive techniques, fast-track protocols, and multimodal analgesic techniques, multimodal analgesia with IV PCA has been shown to be comparable to epidural analgesia in postoperative pain control after abdominal surgery [3].
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS with HIPEC) causes severe postoperative pain due to the long incision from the xiphoid process to the pelvic cavity and the severe invasiveness of the surgery, despite the use of IV PCA [7]. However, the effect of the transabdominal plane (TAP) block with opioid-reduced multimodal IV PCA on the efficacy of the analgesic in patients with severe surgical pain, such as CRS with HIPEC, remains to be investigated. We hypothesized that the addition of a four-quadrant TAP (4Q-TAP) block [8], which is both a bilateral subcostal TAP block and a bilateral lateral TAP block, in patients who underwent CRS with HIPEC using multimodal IV PCA would effectively control severe postoperative pain and enhance postoperative recovery.
The purpose of this randomized double-blinded controlled study was to investigate whether the 4Q-TAP block could effectively reduce the opioid requirement in patients undergoing elective CRS with HIPEC using multimodal IV PCA, and whether it could be beneficial for postoperative recovery.
MATERIALS AND METHODS
Patients
After an Institutional Review Board approved this prospective randomized double-blinded controlled study (no. 2020-11-022-002), 72 adult patients aged 26–81 years, with an American Society of Anesthesiologists physical status ≤ III, and who were scheduled to undergo elective CRS with HIPEC under general anesthesia provided informed consent to receive the 4Q-TAP block and were enrolled in the study. Patients who had local infections or coagulation abnormalities, could not communicate, were drug-addicts, or were using opioids by various routes before surgery were excluded from the study.
Randomization, allocation, and blinding
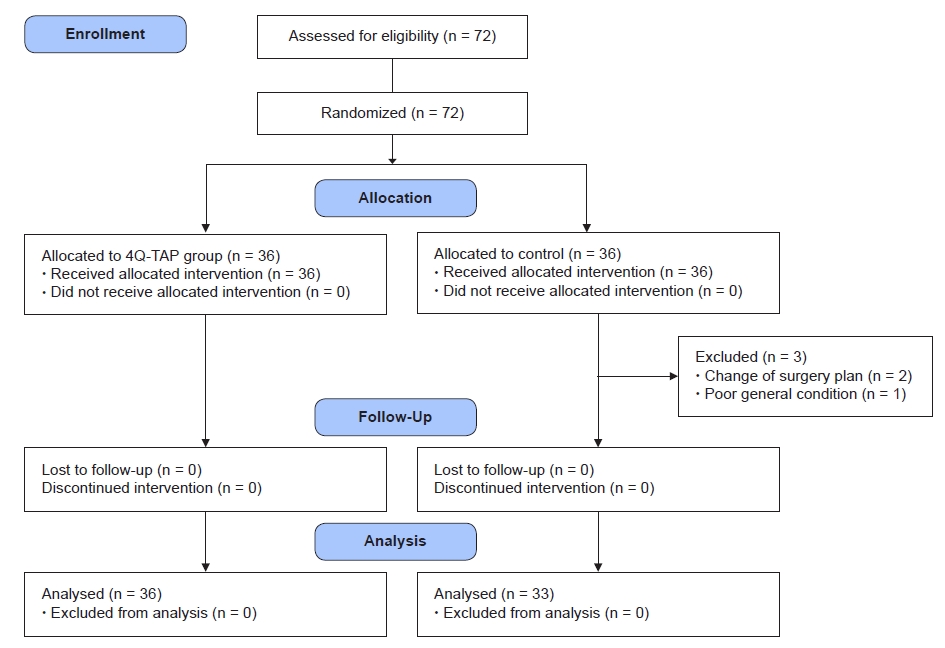
On the day of the surgery, the 72 patients were randomly assigned to either the 4Q-TAP group or the control group using a computer-generated randomization table. We used a block randomization method where block sizes of 2 and 4 were also randomly mixed. We used sealed, opaque, sequentially numbered envelopes that were opened by a single investigator when the patient arrived at the operating room (Fig. 1).
The 4Q-TAP group received a postoperative, ultrasound-guided, four-quadrant TAP block, and the control group received an equivalent volume of normal saline. The same type of syringe was used for administering the study drugs; the color of the syringe and that of the study drugs was identical, and eventually the syringes containing the study drugs were indistinguishable. A designated independent investigator prepared four syringes containing equal volume mixtures of 0.75% ropivacaine and 2% lidocaine (10 ml × 4) or normal saline (10 ml × 4) according to the assignment, and did not participate in any other study processes. All patients, surgeons, anesthesiologists, and follow-up observers were blinded to the assignment.
Anesthesia
The patients were taken to the operating room where standard monitors were applied, including pulse oximetry, electrocardiography, blood pressure and temperature monitoring, capnography, and end-tidal gas analysis. Before the induction of anesthesia, all patients were asked about experiencing motion sickness, smoking history, and current smoking habits. Anesthesia was performed by an attending anesthesiologist. None of the patients received any premedication. Total intravenous anesthesia with propofol and remifentanil was administered to the patients, the blood pressure was adjusted to approximately 20% of the preoperative blood pressure, and the bispectral index level was maintained between 40 and 60. Neuromuscular blockage was performed with 0.8 mg/kg of rocuronium iv, and for sustained relaxation, 5 µg/kg/min of rocuronium was infused continuously. The lungs were ventilated with O2 air to maintain normocapnia with a basal positive end-expiratory pressure of 5–7 cmH2O. All patients underwent goal-directed fluid therapy wherein the total volumes of fluids and transfusion, urinary output, and estimated blood loss were examined. To avoid surgical site infections, antibiotics were administered 1 h before skin incision, and 30 mg of ketorolac and 5 mg of dexamethasone were administered to relieve excess abdominal inflammation, if not contraindicated. Ketorolac was not used in patients with elevated creatinine concentrations to avoid the possibility of acute kidney injury in perioperative patients [9]. Just before HIPEC, 2 g of propacetamol was prophylactically administered to avoid profound systemic hyperthermia. HIPEC was performed for 90 min in all patients. On completion of HIPEC, the abdominal cavity was opened again, and the surgical field was checked for bleeding or injury due to HIPEC. The abdomen was finally closed, and stoma formation was performed as necessary.
After suturing, all patients received ultrasound-guided bilateral TAP block from an experienced anesthesiologist. After the block, the neuromuscular blockade was reversed, and the endotracheal tube was extubated. IV PCA (AutoselectorⓇ, ACE Medical Co., Ltd., Korea) containing fentanyl (0.1 µg/kg/ml), nefopam (60 mg), and lidocaine (400 mg) in 100 ml was applied to the patients, and maintenance infusion was started at 2 ml/h in both groups and titrated appropriately to the pain level. Considering the postoperative pain period of 4–5 days, the total amount of IV PCA was prepared at 200 ml for the 4Q-TAP group and 300 ml for the control group. The programmed bolus dose was 1 ml and the lock-out time was 15 min. The routine postoperative use of NSAIDs was prohibited because of the risk of acute kidney injury after CRS [9].
In the PACU, recovery profiles and pain scores of all patients were measured, and 0.1 mg/kg of oxycodone [10] was administered as a rescue analgesic when the numerical rating scale (NRS) was 5 or higher. The number of rescue analgesics and the incidence of complications such as nausea, dizziness, sedation, or hypotension were observed. Patients who experienced hypotension or excessive bleeding during surgery were transferred to the intensive care unit. 4Q-TAP block.
The skin was prepared for sterilization, and the position of the transverse abdominis muscle was confirmed using ultrasound. Due to peritonectomy, the peritoneal line was not well differentiated and the intestine was often in an ileus state with little movement; therefore, the correct block point was carefully confirmed by moving the probe back and forth widely. First, the position of the transversus abdominis muscle was confirmed in the midaxillary line between the costal margin and the iliac crest. Then, the linear probe was moved forward until it met the rectus abdominis muscle. Between the transversus abdominis and rectus abdominis muscles, bilateral subcostal TAP blocks were performed with a 10 ml mixture of 2% lidocaine and 0.75% ropivacaine per site. The operator carefully inserted the needle using the in-plane ultrasound technique to avoid bowel perforation. When the target point was reached, 0.5 ml of the mixture was injected after confirming the negative pressure. If there was a spread of local anesthetics between the target muscles, the remaining 9.5 ml was injected. For the lateral approach, the probe was positioned in the midaxillary line between the costal margin and iliac crest, and the same amount of drug was injected between the transversus abdominis and internal oblique muscles. If the lateral approach in the midaxillary line was challenging due to stoma formation or drainages, the probe was moved slightly to the rear.
Outcome measurements
The primary endpoint was less than 3 times of the rescue analgesic administration (%) for 5 postoperative days. Secondary endpoints included fentanyl doses of IV PCA every 6 h for 5 days, oxycodone requirement in PACU, total opioid consumption converted to morphine milligram equivalent (MME), local anesthetic toxicity, hospital stay, and postoperative morbidity and mortality.
Maintenance doses of IV PCA were started at 2 ml/h and titrated according to the patients’ NRS and complications, if any. When a patient's NRS was 5 or higher and not controllable with the IV PCA bolus, the patient was administered 25 mg of pethidine or 50 mg of tramadol as rescue analgesics. If a patient was calm during sleep, they were not disturbed and were evaluated at NRS of < 5.
Despite the use of the bolus injection, if the patient’s NRS score was higher than 5 and they frequently requested additional rescue analgesics, we increased the dose of IV PCA by 1 ml/hr. If the patient’s NRS score was less than 3, or they had opioid-related side effects such as sedation, dizziness, nausea, retching, or vomiting, we reduced the maintenance dose of IV PCA to 1 ml/h. If opioid-related side effects persisted without improvement, IV PCA was temporarily stopped, and symptoms were observed. When symptoms subsided and an NRS score higher than 5 was reached, IV PCA infusion was restarted at 1 ml/h.
The total frequency of additional analgesic administration for 5 postoperative days was recorded. The NRS scores and maintenance dose of IV PCA were also recorded every 6 h for 5 postoperative days. The IV PCA duration was checked, and if it was stopped, the reason and the time of stopping were recorded. Any adverse events associated with TAP block or IV PCA, hospital stay, and postoperative morbidity and mortality were recorded. As an index of the recovery profile following surgery, postoperative morbidity scores were evaluated in nine domains including infectious, pulmonary, renal, gastrointestinal, cardiovascular, hematological, wound, neurological, and pain problems at 3, 5, 8, 15, and 30 days postoperatively [11]. Pulmonary complications were evaluated based on the requirement of supplemental oxygen, and the infectious domain was evaluated by the current use of antibiotics or body temperature of > 38°C. Renal complications were evaluated by oliguria, increased serum creatinine (> 30% of baseline), or urinary catheter placement, while the gastrointestinal domain was evaluated by an inability to tolerate an enteral diet, including a feeding tube, for any reason. A case wherein a normal diet could not be processed even on the 5th day after CRS with HIPEC was defined as prolonged postoperative ileus, and its incidence was also compared. Cardiovascular domains were evaluated with the following criteria: de novo myocardial infarction or ischemia, hypotension requiring drugs or fluids, arrhythmia, pulmonary edema, or thromboembolic events. Neurological deficits, including confusion and delirium, wound problems, and bleeding requiring blood transfusion were evaluated in each domain. Surgical pain requiring parenteral opioids or regional blocks was also evaluated in the pain domain.
Sample size estimation
The primary outcome was the percentage of patients who received less than three rescue analgesics administered for 5 postoperative days. Seventy-two patients were required to have an 80% chance of detecting, as significant at the 5% level, an increase in the primary outcome measure from 20% in the control group to 50% in the 4Q-TAP group.
Statistical analysis
Data are presented as mean ± SD or number of patients (%). Nonparametric data are presented as median (1Q, 3Q). Quantitative variables were analyzed using the independent t-test or Mann–Whitney U test after normality assumption test with Kolmogorov–Smirnov test. Qualitative variables were analyzed using the chi-square test or Fisher’s exact test. Repeated measure of analysis of variance (RM ANOVA) was performed for repeatedly measured data such as NRS and the amount of fentanyl in IV PCA. All data were analyzed using SPSS® software (version 26.0, IBM Co., USA) and GraphPad Prism (version 7.05, GraphPad Software, USA). Statistical significance was set at P < 0.05.
RESULTS
Study patients
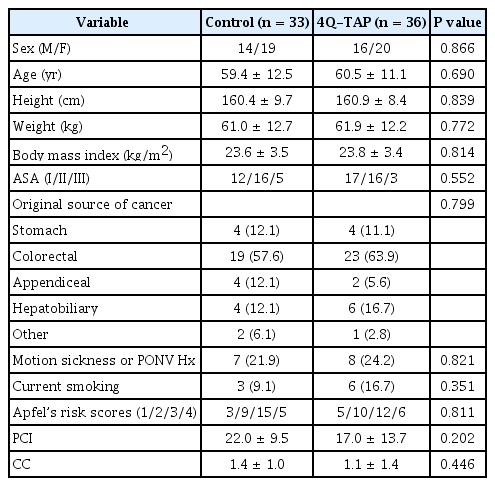
A total of 72 patients were approached for the study and randomized into two groups (Fig. 1). Three patients in the control group were excluded from the study. In 2 patients, palliative surgery was performed instead of CRS, and 1 patient had no CRS and no HIPEC due to poor general condition. Consequently, 36 patients in the 4Q-TAP group and 33 patients in the control group participated in this study. There was no significant difference in the demographic characteristics, Apfel’s postoperative nausea and vomiting (PONV) risk scores, and cancer origin (Table 1) [12,13].
Intraoperative variables
Peritoneal carcinomatosis index (PCI) scores and completeness of cell reduction were not different between the two groups. Estimated blood loss was significantly higher in the control group than in the 4Q-TAP group (400 ml vs. 350 ml, median) (P = 0.036). However, the operation time and anesthetic time were similar and other intraoperative variables including fluid management, transfusion, and urinary output were not different (Table 2).
Analgesic control
RM ANOVA was performed to compare the two groups of NRS scores measured repeatedly every 6 h for 5 postoperative days. However, the sphericity assumption was not satisfied (P < 0.001, Mauchly's sphericity test); hence, the degrees of freedom were adjusted with the Greenhouse-Geisser sphericity correction (adjustment factor = 0.484) [14,15]. No significant difference was observed in the two groups for the NRS scores measured every 6 h for 5 postoperative days. (RM ANOVA with Greenhouse-Geiser sphericity correction, between-subject effect, P = 0.407). The NRS score was significantly different with the passage of time (within-subject effect, P = 0.008), but there was no interaction effect between time and group (P = 0.686) (Fig. 2).
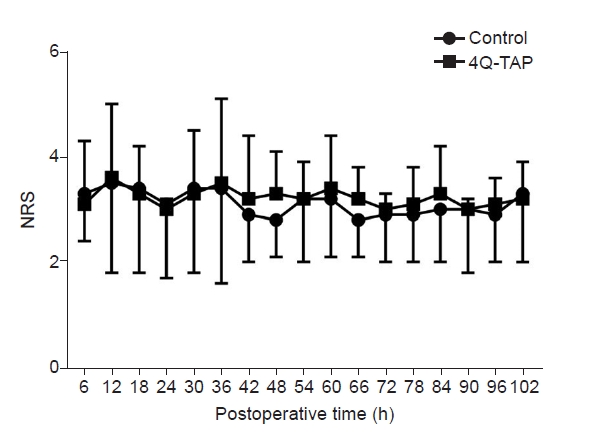
Numeric rating scale (NRS) every 6 h for 5 postoperative days. Repeatedly measured NRS for 5 postoperative days was not different for both the groups (repeated measures of analysis of variance with Greenhous-Geisser correction, between-subject effect, P = 0.407, within-subject effect, P = 0.008, and interaction effect, P = 0.686). 4Q-TAP: four-quadrant transabdominal plane.
Rescue analgesic consumption was divided into the PACU and the ward. In the PACU, the 4Q-TAP group had a significantly smaller oxycodone requirement compared to the control group. The dose of oxycodone was mean ± SD, 0.04 ± 0.07 mg/kg in 4Q-TAP group and 0.09 ± 0.08 mg/kg in control group (P = 0.011). The number of patients who did not receive analgesics in the PACU was also significantly higher in the 4Q-TAP group (24 patients, 66.7%) than in the control group (10 patients, 30.3%) (P = 0.003). However, in the ward, the total frequency of rescue analgesic administration < 3 times for 5 postoperative days was not different between the two groups (24 patients [66.7%] in 4Q-TAP group vs. 15 patients [45.5%] in the control group, P = 0.076).
The administration of fentanyl dose in IV PCA was also analyzed using the RM ANOVA. It also did not satisfy the sphericity assumption (P < 0.001, Mauchly's sphericity test), and the degree of freedom was adjusted with the Greenhouse-Geisser sphericity correction (Adjustment factor = 0.203) [14]. The fentanyl dose administered by IV PCA for 5 postoperative days was significantly lower in the 4Q-TAP group than in the control group (RM ANOVA with Greenhouse-Geiser sphericity correction, between-subject effect, P = 0.038). The fentanyl dose in IV PCA was significantly different with the passage of time (within-subject effect, P < 0.001), and there was also a significant interaction effect between the two variables (P = 0.029) (Fig. 3). A significant dose reduction of fentanyl in IV PCA of the 4Q-TAP block was maintained for 36 h after surgery (Fig. 3).
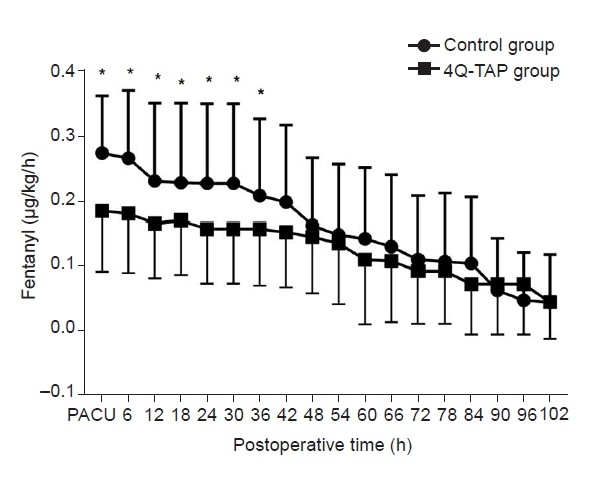
Fentanyl requirement in intravenous patient-controlled analgesia (IV PCA) for 5 postoperative days. Fentanyl doses administered by IV PCA for 5 days after surgery were significantly lower in the 4Q-TAP group than in the control group (repeated measures of analysis of variance with Greenhous-Geisser correction, between-subject effect, P = 0.038). Moreover, fentanyl doses were significantly different according to the time (within-subject effect, P < 0.001), and there was also a significant interaction effect of both variables (P = 0.029) (4Q-TAP group: four-quadrant transabdominal plane block group, Control group: control group). *P < 0.05 in the comparison between two groups at each point of time.
To specify the effects of the 4Q-TAP block, we converted all administered opioid doses, including IV PCA and rescue analgesics to MME per kilogram [16]. Total MME/kg for 5 postoperative days in the 4Q-TAP group was 2.35 (1.63, 3.27) mg/kg and significantly smaller than 3.21 (2.28, 4.31) mg/kg in the control group (Mann–Whitney U test, P = 0.009).
Consequently, the 4Q-TAP group effectively reduced total opioid consumption during the 5 days after CRS with HIPEC.
Adverse events and outcomes
Hospital stay and mortality rates were not significantly different between the two groups (Table 3).
In the postoperative morbidity score, not all domains of postoperative complications differed between the groups (Table 3). The incidence of pulmonary morbidity was similar between the groups. There was no difference in the duration of supplementary oxygen use (6.3 vs. 7.5 days, P = 0.348), and no patient in either group required ventilator care for 5 postoperative days.
The incidence and duration of gastrointestinal morbidity were not significantly different between the two groups (Table 3). Time of liquid diet and regular diet were similar in both groups and prolonged postoperative ileus also occurred at a similar frequency (4Q-TAP, 33.4% vs. Control, 36.0%) in both groups.
The total period of parenteral opioid administration was not different between the groups (mean, 15.3 vs. 16.9 days). Local anesthetic toxicity, such as seizures, metallic taste, blurred vision, ear fullness, or fatal arrhythmia, did not occur in either group.
During 5 days after the surgery, there was no significant difference between the groups in the incidence of IV PCA-related adverse events, including sedation, PONV, dizziness, headache, and heartburn (Table 4). Sedation developed in 2 patients in both the groups. PONV within 24 h developed in 8 patients (22.2%) in the 4Q-TAP group and 4 patients (12.1%) in the control group (P = 0.269). The overall incidence of nausea for 5 days was higher in the 4Q-TAP group (33.3%, 12 patients) than in the control group (18.2%, n = 6), but the difference was not significant (P = 0.152).
The incidence of temporary clamping of IV PCA was 6.1% in the control group and 22.2% in the 4Q-TAP group (P = 0.057), but most of them used IV PCA again without further side effects. The PCA duration was shorter in the control group (89.8 ± 37.1 h) than in the 4Q-TAP group (97.1 ± 38.1 h), but the difference was not statistically significant.
DISCUSSION
In the present study, the addition of a 4Q-TAP block to multimodal IV PCA effectively enhanced postoperative analgesia in patients undergoing CRS with HIPEC.
Postoperative analgesia is an essential part of ERAS. Introduction of the ERAS protocol has also been reported to be related to an increase in survival rates of patients with CRS with HIPEC [1]. Particularly, the use of an opioid-sparing regimen for pain management is emphasized to avoid delayed recovery and multimodal analgesia techniques, including various drugs and epidural analgesia [2].
However, epidural analgesia should be used with caution in patients with CRS and HIPEC. Although not many cases are reported of coagulation abnormalities that are insufficient to maintain and remove the epidural catheter after surgery [17], but massive bleeding may occur during the surgery, according to the severity or invasiveness of the cancer. Hurdle et al. [18] reported that postoperative coagulopathy occurred in approximately 40% of patients who underwent CRS with HIPEC, and the incidence was higher with intraoperative blood transfusion or higher PCI scores. Therefore, careful attention should be given to the management of epidural catheters in high-risk patients. However, since it is difficult to predict the patient's PCI score and bleeding volume before surgery, it is difficult to guarantee the safety of preoperative epidural catheterization. Moreover, to cover the long dermatome of CRS with HIPEC [19], the dose of local anesthetics for epidural analgesia should be greatly increased. Hemodynamic stability, which is an advantage of thoracic epidural analgesia, is difficult to expect and thus hypotensive complications are inevitable. Poorer analgesia compared to a well-controlled IV PCA may also reduce patient satisfaction [3]. Some studies have shown that thoracic epidural analgesia increased the complexity of recovery and rather increased the total hospital stay [20]. These studies explained that if epidural analgesia were performed in surgeries with short recovery periods, the start of anticoagulation after surgery was delayed, and the voiding rehabilitation period due to Foley catheter placement would be also required, which in turn delayed the recovery.
Recently, TAP blocks have been implemented as a new alternative to epidural analgesia [21]. However, in CRS with HIPEC, postoperative pain is extremely severe and has a long duration. The pain not only is because of the abdominal wall but also manifests through various mechanisms such as inflammatory, neuropathic pain, visceral, and positional back pain. We also believe that the TAP block alone was insufficient to replace the epidural block. Cata et al. [22] reported that 4Q-TAP block after CRS with HIPEC did not delay recovery profiles, but significantly increased opioid consumption compared with thoracic epidural analgesia. Therefore, we decided to implement multimodal analgesia with low-dose fentanyl, nefopam, lidocaine, NSAIDs, steroids, and a 4Q-TAP block.
The transversus abdominis plane is a potential anatomical space between the transversus abdominis and the internal oblique muscles [23]. Therefore, the TAP block is a kind of “field block” by infiltration of TAP with local anesthetics, not a specific nerve block. There are several routes to block the abdominal walls, including the subcostal, lateral, posterior, and oblique subcostal approaches [23].
The 4Q-TAP block is a bilateral dual TAP block. Borglum et al. first introduced the “four-point approach,” [21] and Niraj et al. [8] also called it the “four-quadrant” TAP block. The 4Q-TAP block, which technically combines subcostal with lateral or posterior TAP blocks, provides a wider coverage of the upper and lower abdominal walls. Some studies have shown that the posterior TAP block and the posteromedial quadratus lumborum block were more effective than the lateral TAP block [24]. However, in this study, the procedure was performed in the lithotomy position in the operating room, and a lateral TAP block was preferred for positional merit in our study.
The implementation of TAP block in patients with CRS with HIPEC requires careful attention. Due to extensive peritonectomy, the peritoneal lining was unclear, and the remaining intestine was almost in an ileus state and edematous. Therefore, it is often difficult to distinguish between the abdominal anatomical layers. Additionally, it was challenging to secure the location of the block because of various drains and stoma formations. To safely perform the block, it was started from the lateral wall where the three abdominal muscle layers of the external oblique, internal oblique, and transversus abdominis muscles were clearly visible, then moved along the subcostal line to the point where the internal oblique muscle disappeared and met the rectus abdominis muscle. At that point, the subcostal TAP block was performed with 10 ml of an equal volume mixture of 0.75% ropivacaine and 2% lidocaine. The same dose of an equal volume mixture of ropivacaine and lidocaine was also injected in the lateral TAP block site. The procedure was repeated on the other side.
A field block or a plane block, such as TAP, may have a satisfactory effect when a sufficient volume is injected. Generally, it is recommended to inject at least 15 ml of a single TAP block. According to a pilot study [25], in the case of the same dose of local anesthetics, even if the volume increased by changing the concentration, the height of the dermatomal blockade could not be increased, but rather the block duration was decreased. When multiple blocks are required, safe dose selection is important and should be calculated in advance. We mixed 0.75% ropivacaine and 2% lidocaine in a 1:1 ratio to reduce systemic toxicity and to accelerate the onset. Generally, the median effective analgesic dose (ED50) of ropivacaine for TAP block is approximately 2.7 mg/kg, which is close to the toxic concentration (3 mg/kg) [26]. In our study, all patients received ropivacaine (37.5 mg) per site, and the total dose of ropivacaine was 150 mg.
It has been reported that the TAP block duration of long-acting local anesthetics is 6–24 h with no definite preemptive analgesic effects [27]. In this study, we used a local anesthetic mixture, not just a long-acting one. An equal volume mixture has been reported to have a shorter duration owing to the diluted concentration of local anesthetics [27]. The 4Q-TAP group required a significantly smaller amount of rescue analgesics in the PACU than the control group. We did not examine the exact onset time of 4Q-TAP block, but the reduced opioid requirement in the PACU suggests the influence of the nerve block. After the PACU, rescue analgesic consumption was not different, but the IV PCA requirement was significantly reduced in the 4Q-TAP group and stable pain control was achieved by IV PCA maintenance.
Although total opioid usage was reduced with the TAP block, opioid-related complications such as PONV, dizziness, sedation, or prolonged postoperative ileus were not different between the groups. Although not statistically significant, the incidence of PONV was slightly higher in the 4Q-TAP group from the day after surgery, and the number of transient PCA clamps was also higher in the 4Q-TAP group (22.2%) compared to the control group (6.1%) (P = 0.058). A meta-analysis study by Zhao et al. [28] also reported that PONV significantly increased after the TAP block in laparoscopic intestinal surgery. The increased PONV was presumed to be caused by a temporary opioid overdose because the IV PCA dose titration was not performed quickly for the fear of pain. From the day after surgery, IV PCA dose reduction may be necessary in patients with a TAP block, and further prospective studies on IV PCA maintenance doses with TAP blocks are needed.
Additionally, despite the opioid-reducing effect in the 4Q-TAP group, there was no significant difference in the overall outcome or morbidity compared to the control group. Various multimodal analgesic regimens have enhanced recovery by lowering the opioid requirement to increase gastrointestinal motility, reducing pulmonary complications, and promoting ambulation [4,29]. In this study, no recovery outcomes, including hospital stay and various morbidities were different. We speculate that this might be because various analgesic drugs such as lidocaine, NSAIDs, steroids, or nefopam were used together in all patients. Moreover, although the opioid dose of IV PCA in the control group was higher than that in the TAP group, the total amount of opioids administered was not large enough to delay the recovery, even in the control group.
This study has some limitations. First, we titrated the IV PCA according to the NRS score and the presence of complications. Rescue analgesics and IV PCA could be administered in a complementary manner. Therefore, this may have been another intervention other than the 4Q-TAP block. To eliminate this effect, we calculated MME of total opioid use and compared the difference between the two groups. Second, we did not examine the plasma concentrations of long-acting local anesthetics in the 4Q-TAP group. All patients were administered ropivacaine (150 mg). Approximately 15.2% of the 4Q-TAP group exceeded the toxic concentration of ropivacaine (approximately 3 mg/kg). However, owing to the nature of the field block, injecting a smaller dose per site may lead to block failure, and diluted concentrations lead to a shorter duration of block. Moreover, the addition of a small dose of 2% lidocaine in IV PCA also has the potential to augment the effect of systemic toxicity of local anesthetics. International guidelines for the safe use of parenteral lidocaine have not yet been developed, however, the recently published international consensus statement also recommends that parenteral lidocaine should be used at an interval of 4 h after nerve block [30]. Fortunately, there was no systemic toxicity in this study, but it would be influenced by the small sample size and would not guarantee safety. Further studies on the safe dose and various methods to reduce the systemic toxicity of multiple TAP blocks are needed. Clinicians performing multiple TAP blocks should always be thoroughly prepared for the risk of systemic toxicity of local anesthetics.
In conclusion, the addition of ultrasound-guided 4Q-TAP block with multimodal IV PCA effectively reduced total opioid requirements in the postoperative period in patients who underwent CRS with HIPEC. However, it did not change recovery outcomes and hospital stay.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Sung Mi Ji, Min A Kwon. Formal analysis: Min A Kwon. Methodology: Min A Kwon. Writing - original draft: Jae Gyok Song, Nayoung Choi. Writing - review & editing: Min A Kwon. Investigation: Jae Gyok Song, Nayoung Choi, Minji Kang, Min A Kwon. Resources: Sung Mi Ji, Dong-wook Kim. Software: Minji Kang. Supervision: Dong-wook Kim, Min A Kwon.