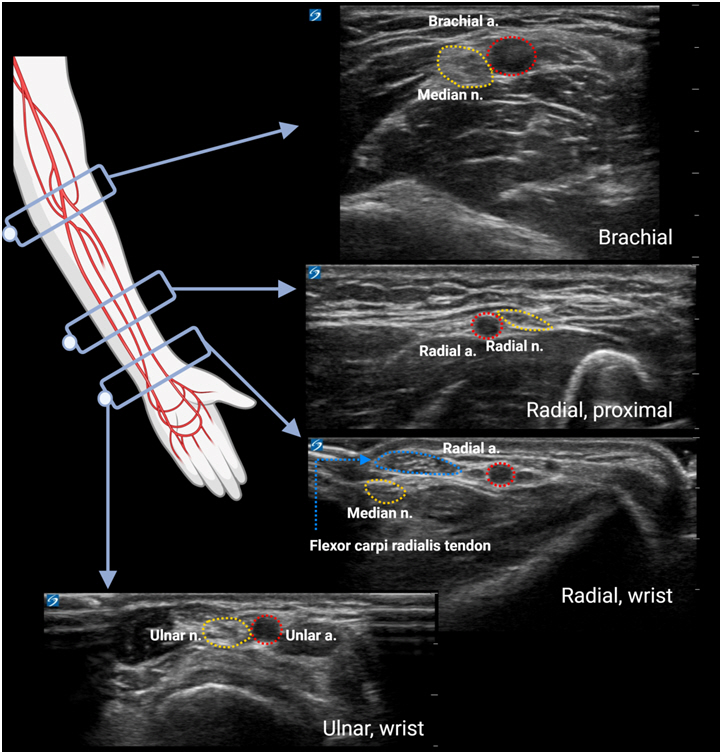
1. Ishii S, Shime N, Shibasaki M, Sawa T. Ultrasound-guided radial artery catheterization in infants and small children. Pediatr Crit Care Med 2013; 14: 471-3.


2. Kim JM, Arakawa K, Bliss J. Arterial cannulation: factors in the development of occlusion. Anesth Analg 1975; 54: 836-41.


3. Latham GJ, Bosenberg AT, Low DK. Images in anesthesiology: radial artery spasm in an infant as documented by high-frequency micro-ultrasound. Anesthesiology 2014; 120: 1254.


4. Nuttall G, Burckhardt J, Hadley A, Kane S, Kor D, Marienau MS, et al. Surgical and patient risk factors for severe arterial line complications in adults. Anesthesiology 2016; 124: 590-7.


6. Beniwal S, Bhargava K, Kausik SK. Size of distal radial and distal ulnar arteries in adults of southern Rajasthan and their implications for percutaneous coronary interventions. Indian Heart J 2014; 66: 506-9.

7. Ashraf T, Panhwar Z, Habib S, Memon MA, Shamsi F, Arif J. Size of radial and ulnar artery in local population. J Pak Med Assoc 2010; 60: 817-9.


8. Kim EH, Lee JH, Song IK, Kim JT, Lee WJ, Kim HS. Posterior tibial artery as an alternative to the radial artery for arterial cannulation site in small children: a randomized controlled study. Anesthesiology 2017; 127: 423-31.


10. Min SW, Cho HR, Jeon YT, Oh AY, Park HP, Yang CW, et al. Effect of bevel direction on the success rate of ultrasound-guided radial arterial catheterization. BMC Anesthesiol 2016; 16: 34.

11. Tiru B, Bloomstone JA, McGee WT. Radial artery cannulation: a review article. J Anesth Clin Res 2012; 3: 209.


12. Lamperti M, Biasucci DG, Disma N, Pittiruti M, Breschan C, Vailati D, et al. European Society of Anaesthesiology guidelines on peri-operative use of ultrasound-guided for vascular access (PERSEUS vascular access). Eur J Anaesthesiol 2020; 37: 344-76.


13. Bertrand OF, Carey PC, Gilchrist IC. Allen or no allen: that is the question! J Am Coll Cardiol 2014; 63: 1842-4.


14. Riekkinen HV, Karkola KO, Kankainen A. The radial artery is larger than the ulnar. Ann Thorac Surg 2003; 75: 882-4.


16. Tomiyama Y, Yoshinaga K, Fujii S, Ochi N, Inoue M, Nishida M, et al. Accurate quantitative measurements of brachial artery cross-sectional vascular area and vascular volume elastic modulus using automated oscillometric measurements: comparison with brachial artery ultrasound. Hypertens Res 2015; 38: 478-84.


17. Kim EH, Lee HC, Chung J, Ji SH, Jang YE, Lee JH, et al. Flow-mediated dilatation of the brachial artery for assessing endothelial dysfunction in children with Moyamoya disease. Pediatr Neurosurg 2020; 55: 149-54.
18. Khan ZA, Khan MA, MohammednourAltaf F, Alkhushi AG, Alasmari WA. Diameter of the dorsalis pedis artery and its clinical relevance. IOSR-JDMS 2016; 15: 129-33.


21. Sun J, Ding Z, Qian Y, Peng YG. Central-radial artery pressure gradient after cardiopulmonary bypass is associated with cardiac function and may affect therapeutic direction. PLoS One 2013; 8: e68890.


23. Rudnick MR, Marchi LD, Plotkin JS. Hemodynamic monitoring during liver transplantation: a state of the art review. World J Hepatol 2015; 7: 1302-11.


24. Spector KS, Lawson WE. Optimizing safe femoral access during cardiac catheterization. Catheter Cardiovasc Interv 2001; 53: 209-12.


25. Bhatia N, Sivaprakasam J, Allford M, Guruswamy V. The relative position of femoral artery and vein in children under general anesthesia--an ultrasound-guided observational study. Paediatr Anaesth 2014; 24: 1164-8.


26. Rajebi H, Rajebi MR. Optimizing common femoral artery access. Tech Vasc Interv Radiol 2015; 18: 76-81.

27. Troianos CA, Hartman GS, Glas KE, Skubas NJ, Eberhardt RT, Walker JD, et al. Councils on Intraoperative Echocardiography and Vascular Ultrasound of the American Society of Echocardiography. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2011; 24: 1291-318.


28. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth 2016; 116: 610-7.

29. Seto AH, Roberts JS, Abu-Fadel MS, Czak SJ, Latif F, Jain SP, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (radial artery access with ultrasound trial). JACC Cardiovasc Interv 2015; 8: 283-91.

30. Jang YE, Kim EH, Lee JH, Kim HS, Kim JT. Guidewire-assisted vs. direct radial arterial cannulation in neonates and infants: a randomised controlled trial. Eur J Anaesthesiol 2019; 36: 738-44.


31. Abu-Omar Y, Mussa S, Anastasiadis K, Steel S, Hands L, Taggart DP. Duplex ultrasonography predicts safety of radial artery harvest in the presence of an abnormal Allen test. Ann Thorac Surg 2004; 77: 116-9.


32. Habib J, Baetz L, Satiani B. Assessment of collateral circulation to the hand prior to radial artery harvest. Vasc Med 2012; 17: 352-61.


33. Chittick P, Russo V, Sims M, Robinson-Dunn B, Oleszkowicz S, Sawarynski K, et al. An outbreak of Pseudomonas aeruginosa respiratory tract infections associated with intrinsically contaminated ultrasound transmission gel. Infect Control Hosp Epidemiol 2013; 34: 850-3.


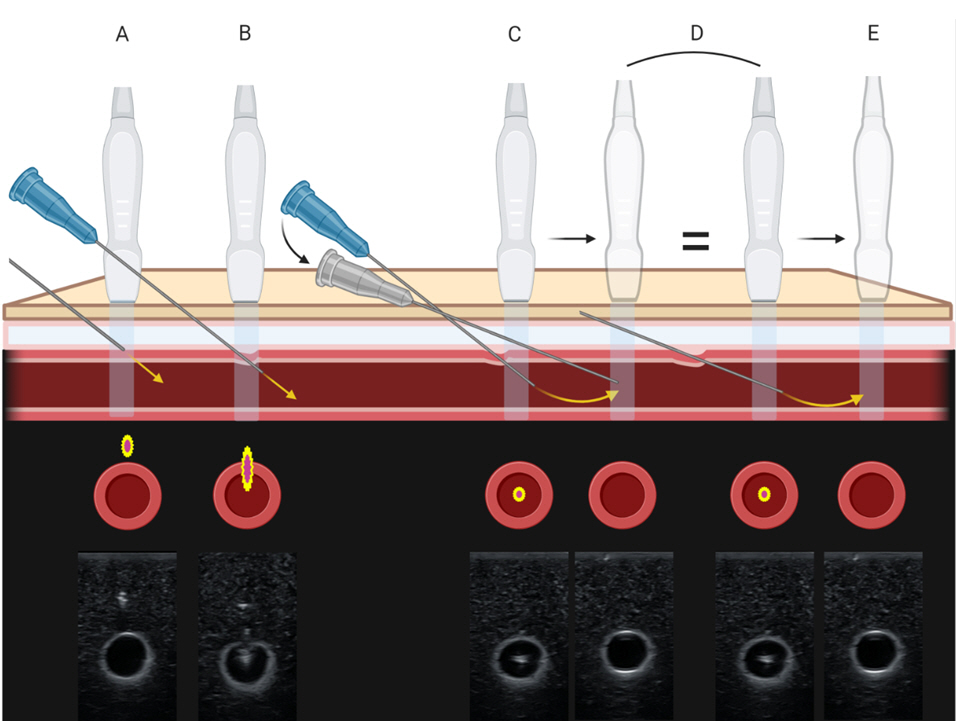
34. Berk D, Gurkan Y, Kus A, Ulugol H, Solak M, Toker K. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput 2013; 27: 319-24.


35. Quan Z, Tian M, Chi P, Cao Y, Li X, Peng K. Modified short-axis out-of-plane ultrasound versus conventional long-axis in-plane ultrasound to guide radial artery cannulation: a randomized controlled trial. Anesth Analg 2014; 119: 163-9.


36. Sethi S, Maitra S, Saini V, Samra T, Malhotra SK. Comparison of short-axis out-of-plane versus long-axis in-plane ultrasound-guided radial arterial cannulation in adult patients: a randomized controlled trial. J Anesth 2017; 31: 89-94.


37. Song IK, Choi JY, Lee JH, Kim EH, Kim HJ, Kim HS, et al. Short-axis/out-of-plane or long-axis/in-plane ultrasound-guided arterial cannulation in children: a randomised controlled trial. Eur J Anaesthesiol 2016; 33: 522-7.


38. Kiberenge RK, Ueda K, Rosauer B. Ultrasound-guided dynamic needle tip positioning technique versus palpation technique for radial arterial cannulation in adult surgical patients: a randomized controlled trial. Anesth Analg 2018; 126: 120-6.


39. Liu L, Tan Y, Li S, Tian J. "Modified dynamic needle tip positioning" short-axis, out-of-plane, ultrasound-guided radial artery cannulation in neonates: a randomized controlled trial. Anesth Analg 2019; 129: 178-83.


40. Nam K, Jeon Y, Yoon S, Kwon SM, Kang P, Cho YJ, et al. Ultrasound-guided radial artery cannulation using dynamic needle tip positioning versus conventional long-axis in-plane techniques in cardiac surgery patients: a randomized, controlled trial. Minerva Anestesiol 2020; 86: 30-7.


43. Buetti N, Ruckly S, Lucet JC, Bouadma L, Schwebel C, Mimoz O, et al. Ultrasound guidance and risk for intravascular catheter-related infections among peripheral arterial catheters: a post-hoc analysis of two large randomized-controlled trials. Ann Intensive Care 2020; 10: 89.


44. Pancholy SB, Coppola J, Patel T. Subcutaneous administration of nitroglycerin to facilitate radial artery cannulation. Catheter Cardiovasc Interv 2006; 68: 389-91.


46. Ezhumalai B, Satheesh S, Jayaraman B. Effects of subcutaneously infiltrated nitroglycerin on diameter, palpability, ease-of-puncture and pre-cannulation spasm of radial artery during transradial coronary angiography. Indian Heart J 2014; 66: 593-7.


47. Chen Y, Ke Z, Xiao J, Lin M, Huang X, Yan C, et al. Subcutaneous injection of nitroglycerin at the radial artery puncture site reduces the risk of early radial artery occlusion after transradial coronary catheterization: a randomized, placebo-controlled clinical trial. Circ Cardiovasc Interv 2018; 11: e006571.

48. Coppola J, Patel T, Kwan T, Sanghvi K, Srivastava S, Shah S, et al. Nitroglycerin, nitroprusside, or both, in preventing radial artery spasm during transradial artery catheterization. J Invasive Cardiol 2006; 18: 155-8.


49. Dharma S, Kedev S, Patel T, Kiemeneij F, Gilchrist IC. A novel approach to reduce radial artery occlusion after transradial catheterization: postprocedural/prehemostasis intra-arterial nitroglycerin. Catheter Cardiovasc Interv 2015; 85: 818-25.

50. Majure DT, Hallaux M, Yeghiazarians Y, Boyle AJ. Topical nitroglycerin and lidocaine locally vasodilate the radial artery without affecting systemic blood pressure: a dose-finding phase I study. J Crit Care 2012 27: 532.e9-13.


51. Beyer AT, Ng R, Singh A, Zimmet J, Shunk K, Yeghiazarians Y, et al. Topical nitroglycerin and lidocaine to dilate the radial artery prior to transradial cardiac catheterization: a randomized, placebo-controlled, double-blind clinical trial: the PRE-DILATE Study. Int J Cardiol 2013; 168: 2575-8.


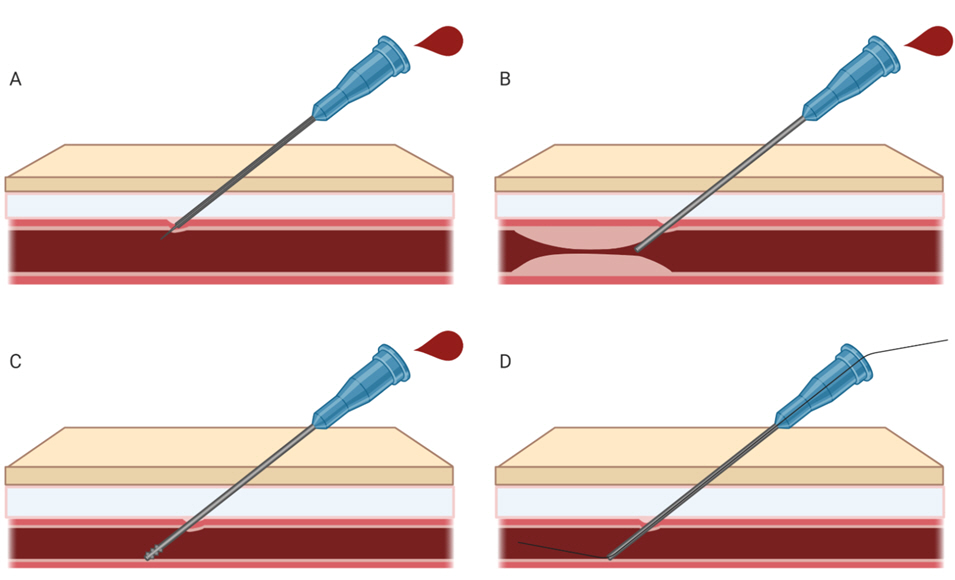
52. Reusz G, Langer C, Jakab L, Morvay Z. Ultrasound-guided vascular access: the importance of the needle bevel. Can J Anaesth 2012; 59: 499-500.


53. Beards SC, Doedens L, Jackson A, Lipman J. A comparison of arterial lines and insertion techniques in critically ill patients. Anaesthesia 1994; 49: 968-73.


54. Yildirim V, Ozal E, Cosar A, Bolcal C, Acikel CH, Kiliç S, et al. Direct versus guidewire-assisted pediatric radial artery cannulation technique. J Cardiothorac Vasc Anesth 2006; 20: 48-50.


55. Lavallée C, Ayoub C, Mansour A, Lambert J, Lebon JS, Lalu MM, et al. Subclavian and axillary vessel anatomy: a prospective observational ultrasound study. Can J Anaesth 2018; 65: 350-9.


56. Melhuish TM, White LD. Optimal wrist positioning for radial arterial cannulation in adults: a systematic review and meta-analysis. Am J Emerg Med 2016; 34: 2372-8.


57. Blanco P. Ultrasound-guided vascular cannulation in critical care patients: a practical review. Med Intensiva 2016; 40: 560-71.


58. Werner SL, Jones RA, Emerman CL. Effect of hip abduction and external rotation on femoral vein exposure for possible cannulation. J Emerg Med 2008; 35: 73-5.

59. Ahn HJ, Lee JW, Yoo SW, Kim JH, Kim KD, Yoo IS, et al. Novel body positioning maximizes femoral venous size in adults: an ultrasonographic evaluation. Hong Kong J Emerg Med 2018; 25: 338-42.


60. Dahl MR, Smead WL, McSweeney TD. Radial artery cannulation: a comparison of 15.2- and 4.45-cm catheters. J Clin Monit 1992; 8: 193-7.

61. Belda FJ, Aguilar G, Teboul JL, Pestaña D, Redondo FJ, Malbrain M, et al. PICS Investigators Group. Complications related to less-invasive haemodynamic monitoring. Br J Anaesth 2011; 106: 482-6.


62. King MA, Garrison MM, Vavilala MS, Zimmerman JJ, Rivara FP. Complications associated with arterial catheterization in children. Pediatr Crit Care Med 2008; 9: 367-71.


64. Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care 2002; 6: 199-204.


65. Baserga MC, Puri A, Sola A. The use of topical nitroglycerin ointment to treat peripheral tissue ischemia secondary to arterial line complications in neonates. J Perinatol 2002; 22: 416-9.


66. Cuper NJ, de Graaff JC, Hartman BJ, Verdaasdonk RM, Kalkman CJ. Difficult arterial cannulation in children: is a near-infrared vascular imaging system the answer? Br J Anaesth 2012; 109: 420-6.


67. Ho HH, Jafary FH, Ong PJ. Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: incidence, predisposing factors, prevention, and management. Cardiovasc Revasc Med 2012; 13: 193-5.


68. Puri VK, Carlson RW, Bander JJ, Weil MH. Complications of vascular catheterization in the critically ill. A prospective study. Crit Care Med 1980; 8: 495-9.


69. Wang A, Hendin A, Millington SJ, Koenig S, Eisen LA, Shiloh AL. Better with ultrasound: arterial line placement. Chest 2020; 157: 574-9.

71. Hambsch ZJ, Kerfeld MJ, Kirkpatrick DR, McEntire DM, Reisbig MD, Youngblood CF, et al. Arterial catheterization and infection: toll-like receptors in defense against microorganisms and therapeutic implications. Clin Transl Sci 2015; 8: 857-70.


74. Rubinos C, Ruland S. Neurologic complications in the intensive care unit. Curr Neurol Neurosci Rep 2016; 16: 57.