 |
 |
- Search
| Anesth Pain Med > Volume 15(4); 2020 > Article |
|
Abstract
Background
Surgeries in patients with coronavirus disease 2019 (COVID-19) put medical staff at a high risk of infection. We report the anesthetic management and infection control of a mechanically ventilated COVID-19 patient who underwent exploratory laparotomy for suspected duodenal ulcer perforation.
Case
A 73-year-old man, mechanically ventilated for confirmed COVID-19, showed clinical and radiographic signs of a perforated duodenal ulcer, and he was transferred under sedation and intubation to a negative-pressure operating room. The operating and assistant staff wore personal protective equipment. High-efficiency particulate absorbing filters were inserted into the expiratory circuits of the anesthesia machine and portable ventilator. No participating staff contracted COVID-19, although the patient later died due to pneumonia.
In December 2019, a novel coronavirus was first identified in Wuhan, China, and the number of infected individuals has since exponentially increased worldwide [1]. The first case in South Korea was reported on January 20, 2020 [2]. The coronavirus disease 2019 (COVID-19) is characterized by a wide range of symptoms, from asymptomatic presentation to mild symptoms, including fever and cough, to severe symptoms, such as acute respiratory distress syndrome, septic shock, and death [1,2]. Studies have shown that ventilatory care is required for 2.3-4.0% of patients with COVID-19 [3,4]. Patients with severe disease are likely to become “super-spreaders,” who shed higher viral loads of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. The exact route of transmission is still unclear, although COVID-19 is known to be transmitted through respiratory droplets and contact transmission. Moreover, a large number of secondary infections have been observed in the hospital setting, similar to those seen with the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [1].
Therefore, when a COVID-19 patient requires surgery, not only are the medical staff required to wear personal protective equipment (PPE), but also additional facilities, such as a negative-pressure operating room, must be available. During general anesthesia of the intubated patient, the risk of aerosol propagation is increased during the connecting and disconnecting of the anesthetic breathing system to the patient’s endotracheal tube as well as with endotracheal suctioning. Furthermore, the infectious material can spread through the endotracheal tube during transportation; thus, special care must be taken for infection control during anesthesia and when transporting the patient.
To date, there are only a few reports on the anesthetic management of patients with severe COVID-19. Herein, we report our experience with anesthetic management and infection control for a COVID-19 patient under ventilatory care who underwent an exploratory laparotomy for a suspected duodenal ulcer perforation. Written informed consent for publication was obtained from the legal guardian of the patient.
A 73-year-old man who was confirmed to have COVID-19 was hospitalized for treatment. The patient had underlying comorbidities, including hypertension and diabetes mellitus, had undergone percutaneous coronary intervention (PCI) two years earlier, and had been taking 100 mg aspirin daily. The patient tested positive for SARS-CoV-2 by real-time reverse transcription polymerase reaction (RT-PCR) on February 27, 2020, and was admitted to the Pohang Medical Center. At admission, the patient presented with a fever of 37.7℃ and cough, which are typical symptoms of COVID-19, and chest radiography revealed ill-defined, hazy, and streaky density in both the lungs (Fig. 1A). Therefore, oral hydroxychloroquine treatment was initiated; however, due to symptoms of pneumonia accompanied by hypotension and melena, the patient was transferred to the Samsung Medical Center for further management. After his vital signs had been stabilized, the patient remained under observation without additional treatment. However, on Day 1 of admission at the Samsung Medical Center, the patient expelled a large quantity of melena and developed hypotension and tachycardia along with a peripheral oxygen saturation (SpO2) of approximately 80%, and his hemoglobin level had decreased to 6.3 mg/dl.
The patient underwent blood transfusion and endotracheal intubation as well as the insertion of arterial catheter and central venous catheter. The patient was evaluated using esophagogastroduodenoscopy (EGD), which revealed a duodenal ulcer without active bleeding. The patient was at risk of re-bleeding; however, computed tomography (CT) and embolization for COVID-19 patients were not possible at the Samsung Medical Center at the time. Therefore, the patient was transferred to our hospital where a COVID-19 patient care environment had been prepared. As the patient was at a high risk of re-bleeding, the patient immediately underwent endoscopic hemoclipping and embolization. Surgery was considered; however, given the patient’s condition and the stress of invasive surgery for the patient, we decided to first perform endoscopy and embolization.
One day after hemoclipping and embolization, the patient complained of abdominal pain, and the follow-up chest X-ray revealed subphrenic free air, an indication of pneumoperitoneum. Based on his endoscopic findings, perforation of the duodenal ulcer was suspected, and emergency surgery was planned. Despite a fever of 37.8℃, the patient’s preoperative vital signs were stable; electrocardiography (ECG) showed a normal sinus rhythm, and chest radiography revealed peripheral lung consolidation, which suggested aggravated pneumonia (Fig. 1B). Blood analysis revealed hemoglobin and C-reactive protein levels of 11.1 mg/dl and 103 mg/L, respectively. Arterial blood gas analysis revealed the following: pH, 7.48; pCO2, 33.0 mmHg; pO2, 173 mmHg; bicarbonate, 24.7 mmol/L; and saturation, 99.4% under mechanical ventilation of synchronized intermittent mandatory ventilation mode with a fraction of inspired oxygen of 0.35. The patient was on a continuous infusion of remifentanil for sedation and intravenous antibiotics, such as tazobactam and piperacillin, to treat the pneumonia.
The patient tested positive for SARS-CoV-2 again when RT-PCR of his nasopharyngeal and oropharyngeal swabs was performed on March 3, 2020. Since the patient presented with severe pneumonia symptoms and persistent detection of the SARS-CoV-2 virus, the risk of nosocomial transmission to the medical staff during endotracheal intubation and mechanical ventilation was high. Therefore, the medical staff who participated in the surgery and anesthesia wore PPE, and the surgery was performed in a negative-pressure operating room. The anesthesiologist and nurse inside the main operating room wore fluid-resistant protective suits, double gloves, boots, shoe covers, and aprons with a powered air-purifying respirator (PAPR) hood (Fig. 2), whereas the staff outside the main operating room wore N95 masks, anteroposterior (vinyl) gowns, and gloves. During the preparations for surgery and anesthesia, the assistant staff, who wore protective suits, double gloves, boots, shoe covers, aprons, N95 masks, and face shields, planned and controlled the patient’s path to the operating room.
After the preparations had been completed, the sedated patient was transferred to the operating room and connected to a portable ventilator equipped with a high-efficiency particulate absorbing (HEPA) filter in the expiratory circuit by medical staff wearing the same PPE who controlled patient’s path. An anesthesiologist and a nurse stood by in the operating room, and the patient was taken over from the accompanying medical staff by the surgeon in the waiting room and subsequently transferred to the operating room through the anteroom (Fig. 3). An assistant surgeon followed the patient and sterilized the site where the cart had moved on the floor by spraying a diluted 1:100 solution of bleach (NaClO 4%). After the patient moved onto the operating table, the stretcher used for patient transfer was similarly disinfected with a diluted 1:100 solution of bleach in the operating room and was again disinfected when it was moved into the anteroom.
Invasive arterial blood pressure (ABP), ECG, heart rate, SpO2, and bispectral index monitoring were commenced in the operating room; the patient’s vital signs were stable. After clamping the endotracheal tube, the tube was detached from the portable ventilator and connected to the anesthesia machine, which had a HEPA filter in the expiratory circuit, and end-tidal carbon dioxide monitoring was started. General anesthesia was induced by 0.5 mg/kg propofol (intravenous) and 0.6 mg/kg rocuronium. Thereafter, the esophageal temperature probe was placed, and temperature monitoring commenced. Anesthesia was maintained by 2.0% sevoflurane and target-controlled infusion of 1.5-3.0 ng/ml remifentanil as well as continuous infusion of 0.15 mg/kg/h rocuronium. Considering the patient’s lung condition, a lung-protective mechanical ventilation strategy (fraction of inspired oxygen, 0.5; fresh gas flow, 2 L/min; tidal volume, 6 ml/kg; peak positive end expiratory pressure, 8 cmH2O cm; and peak inspiratory pressure, ≤ 30 cm H2O) was used.
During surgery, the pressure in the operating room and the anteroom was maintained between −2.5 and −20 Pa. The nurse on standby outside the operating room placed additional required items, such as equipment or drugs, in the anteroom where the item was collected by the medical staff in the main operating room once the outside nurse had left the anteroom. An arterial blood gas sample obtained during surgery was sent out of the operating room in the same way for analysis. The operation lasted 120 min, without any notable events, and the perforated ulcer was found at the expected site. The operative procedure included pyloric exclusion with primary closure of the perforation and formation of a gastrojejunostomy.
The patient was intravenously injected with 0.07 mg/kg midazolam and 0.3 mg/kg rocuronium for sedation during transfer to the ward, while maintaining the intubation. Prior to the detachment of the anesthesia machine from the patient’s tube, closed in-line tracheal suction was performed. Subsequently, the anesthesiologist switched the ventilator to manual mode, opened the adjustable pressure-limiting valve, and clamped the tracheal tube. The patient was transferred to a stretcher cart that was placed in the anteroom. Monitoring of ECG, SpO2, and ABP continued, and 10 L/min of oxygen was supplied through an Ambu bag with a HEPA filter. The patient was transferred to the waiting room after passing through the anteroom and was finally moved to the intensive care unit (ICU) by the surgeon who had, in the meantime, changed the external gloves and footwear (Table 1).
The disposable equipment used for anesthesia was discarded, including the capnography sampling line and water trap, whereas reusable equipment was disinfected with a diluted 1:100 solution of bleach after first remaining in negative-pressure ventilation in the operating room for an hour.
The patient was continuously monitored in the ICU. Antibiotics were administered intravenously, and hydroxychloroquine was administered via L-tube to treat pneumonia. No surgical complications, such as re-bleeding or perforation of the surgical site, were observed. However, despite continued pneumonia treatment up to the fifth postoperative day, elevated C-reactive protein and fever between 37.3 and 38.8℃ persisted, and the patient consistently tested positive for SARS-CoV-2 by RT-PCR. From chest radiography, increased pneumonic infiltration and pleural effusion were observed (Fig. 1C). Other vital signs were stable, except for body temperature. On the sixth postoperative day, the patient developed a fever of 39.5℃, and hypotension was observed. The administration of fluid and norepinephrine was initiated after the pulmonary medicine staff diagnosed septic shock that developed due to failure to control the infection despite the continuous treatment for pneumonia. Despite persistent resuscitation, the patient expired on the seventh postoperative day.
We described the case of an emergency exploratory laparotomy under general anesthesia for suspected duodenal perforation in a patient with a highly infectious respiratory viral disease. For COVID-19 patients, avoiding surgery whenever possible is the best option for the safety of the patient and the medical staff [4,5], particularly when such patients require ventilatory support, as they are more likely to shed more of the infectious agent [3]. However, peptic ulcer perforation is a potentially lethal surgical emergency, wherein the mortality rate increases with every hour of surgical delay [6]. Therefore, we decided to perform surgery despite the infection risk. This patient had stopped taking aspirin for 2 days, which he had been taking every day since undergoing PCI 2 years earlier, and his general condition was exacerbated by aggravated pneumonia secondary to COVID-19. Considering the general condition of the patient and risk of intraoperative bleeding and postoperative re-bleeding, and due to the concern about infecting medical staff with COVID-19, our multidisciplinary team opted to perform an exploratory laparotomy for pyloric exclusion with primary repair of the perforated site and gastrojejunostomy, instead of performing laparoscopic surgery, to minimize the need for further invasive treatment. Although regional anesthesia is preferred over general anesthesia given the infectivity of COVID-19 [4], this operation was performed under general anesthesia because it was difficult to obtain sufficient motor and sensory blockade for the surgical site through regional anesthesia, and the patient was already intubated and receiving ventilatory support.
In this patient, peptic ulcer perforation was diagnosed using portable chest radiography. When a chest X-ray is used to diagnose peptic ulcer perforation, it may not show the exact cause of pneumoperitoneum, as it has only 75% sensitivity [6]. Abdominal CT has a greater sensitivity (up to 98% sensitivity) and is more valuable for differential diagnosis [7]. Nevertheless, we suspected ulcer perforation based on the detection of a duodenal ulcer in the preoperative EGD and subphrenic free air on the chest X-ray. As the patient’s clinical findings supported the portable X-ray findings, and given the time and procedure required for infection control and transport, emergency surgery was immediately performed without additional CT. It is difficult to control the spread of infection when transferring COVID-19 patients; therefore, tests that require patient transfer demand time and resources, which may limit information gathering prior to anesthesia and may delay emergency surgery. As a result, anesthesiologists need to balance the need for preoperative examination and the urgency of the operation.
According to the recommendations of anesthesia management for COVID-19 patients, surgery on COVID-19 patients should be performed in a negative-pressure operating room, with the use of PPE, to prevent the infection of medical staff [3,4]. When this patient was treated, no previous COVID-19 patient had undergone surgery in Korea, and no surgical protocol had been established for COVID-19 patients. Our hospital had established a surgical protocol for MERS-CoV patients during the MERS epidemic in 2015; thus, we planned this surgery based on our MERS protocol and surgical experience with MERS patients. According to this protocol, surgery on MERS-CoV patients should be performed in a negative-pressure isolation room, and all healthcare workers involved in aerosol-generating procedures should wear PPE.
In our hospital, every operating room has an individual ventilation system with a HEPA filtration [8]. The airflow inside the operating room forms a laminar flow from the ceiling and escapes through four prefilters located on the wall, protecting the surgical field from airflow. The operating room was maintained at 12-15 air changes per hour and at a negative-pressure between -2.5 Pa and -20 Pa. To prevent airborne infection, the operating room needs to be maintained at a continuous negative air pressure below -2.5 Pa [9]. Negative-pressure operating rooms in other hospitals often have an anteroom in front of the main operating room, through which medical staff must pass when entering and exiting the operating room [4,10]. If the anteroom is used when medical staff members need to be replaced, for instance, due to prolonged surgery, the room needs to be sterilized, and the space cannot be used while replacing the medical staff and disinfecting the anteroom. In contrast, our main operating room has an additional exit (separate from the anteroom) with a doffing area and shower booth leading to its entrance, so that medical staff can leave the operating room without passing through the anteroom (Fig. 3). With this additional passageway, the anteroom can be kept clean throughout the surgery, allowing external staff to deliver additional equipment via the anteroom.
The anesthesiologist and surgeons who were involved in the surgery all wore PPE, which included a PAPR. An N95 mask may seal leaks and increase resistance to breathing, while a PAPR is comfortable to wear for a relatively long period. However, the disadvantages of a PAPR include the impossibility of auscultation, interference of communication between medical staff due to fan noise, and limited battery power [5], which we experienced during this surgery. There is no evidence that wearing a PAPR during aerosol-generating procedures reduces viral transmission secondary to airborne spread, compared to wearing an N95 mask [3]. Therefore, anesthesiologists must take this into consideration when wearing a PAPR and should fully discuss the anesthesia and surgery plan with the medical team ahead of time to minimize the difficulties that could be caused by communication error.
There was a risk of aerosol propagation as this patient was transferred while maintaining tracheal intubation. In previous cases associated with COVID-19 and MERS, no patient was transferred while maintaining tracheal intubation [11-13]. Our patient’s transfer was conducted after passage control, with the accompanying staff wearing PPE. To prevent unexpected hospital-acquired infection, a HEPA filter was added to the expiratory circuit of the portable ventilator when the patient entered the operating room, and it was applied between the Ambu bag and the tracheal tube when the patient left the room. Recommendations for the transportation of COVID-19 patients state that HEPA filters need to be added to the Ambu bag or portable ventilator [14]. Prior to transfer, it is advisable to sedate the patient using sedatives and neuromuscular blockade agents to prevent aerosol propagation from coughing or spontaneous breathing. Additionally, a dental mask was applied to cover the patient’s mouth. Such preventive measures are considered to be effective for infection control during patient transfer.
Contaminants in the air of the operating room and surfaces of the anesthesia machine and monitoring device should be reliably disinfected after the anesthesia. In our case, after the surgery was completed, the negative-pressure operating room was closed and room ventilation was performed for an hour. When room ventilation is performed for 35 min under the condition that 12 air changes per hour are satisfied, 99.9% of aerosol type contaminants in the operating room are removed [8,9]. The surface of the operating room and surfaces of the devices used for anesthesia were disinfected with a diluted 1:100 solution of bleach, and decomposable parts of the anesthesia machine, such as bellows and adjustable pressure-limiting valves, were sterilized with ethylene oxide. Replaceable items, such as carbon dioxide absorbent and breathing bag, were discarded. In our case, the sampling line and water trap were discarded because the sampling gas from the patient was collected before passing through the HEPA filter. In addition, we considered that the aerosol generated from the COVID-19 patient could contaminate the inside of the anesthesia machine even after passing through the HEPA filter of the expiratory circuit and the filter inside the water trap; the anesthesia machine was covered with vinyl and unused for more than 72 h. SARS-CoV-2 showed no viability after 72 h on surfaces of plastic or steel [15]. Up to 2 months after surgery, medical staff who participated in this surgery showed no significant signs or symptoms of infection, and no nosocomial infections were reported. As COVID-19 spreads rapidly worldwide, it is likely that more cases of severe COVID-19 patients requiring surgery will be encountered. Therefore, clinical guidelines for anesthesia management and infection control during the transportation of severe COVID-19 patients should be established. We hope that this case report will contribute to the establishment of clinical guidelines for the surgical management of patients with COVID-19 and the design of negative-pressure operating rooms.
Fig. 1.
Chest radiograph of the patient. (A) Chest radiograph of the patient showing ill-defined, hazy, and streaky density in both the lungs. (B) Chest radiograph showing subphrenic free air on both sides, homogenous increased density in the right costophrenic angle, and aggravated bilateral peripheral lung consolidation. (C) Chest radiograph showing increased pneumonic consolidation in both lung fields, especially that of the right lower lobe and pleural effusion right.

Fig. 2.
Medical staff in preparation for surgery. The medical staff who were going to be inside the operating room each wore a fluid-resistant gown, PAPR, gloves, boots, and an apron. Staff wore double layers of gloves and boots. The surgeon and scrub nurse wore sterile gowns and surgical gloves on top of the PPE. The surgeons in the picture wore N95 masks inside the PAPR hoods. There is no strong evidence for the added protective effects of the concurrent use of N95 masks with PAPR [4]. Laminar flows from the ceiling toward the operating field (dotted arrow). A: anesthesiologist, S: surgeon, SN: scrub nurse, CN: circulating nurse, PAPR: powered air-purifying respirator, PPE: personal protective equipment.
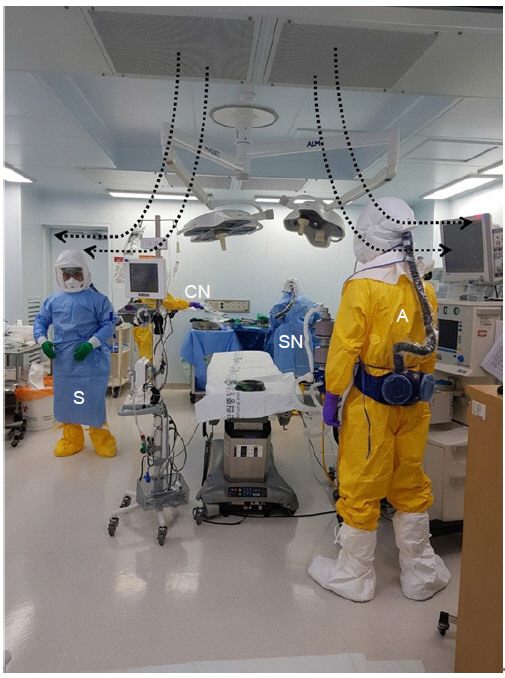
Fig. 3.
Overall design of the negative-pressure operating room. Negative pressure is maintained only in (1) and (2), and when the doors of these rooms are opened, the negative pressure is temporarily lost. Square dotted arrow: pathway for entry of medical staff and patients; round dotted arrow: pathway for the patient’s exit, solid arrow: pathway for the exit of medical staff who do not participate in the patient transfer, overlaid arrow: pathway for medical staff who participate in the patient transfer after changing PPE that was worn during surgery. (1) main operating room, (2) anteroom, (3) waiting room, (4) doffing area, (5) buffer area. PPE: personal protective equipment.
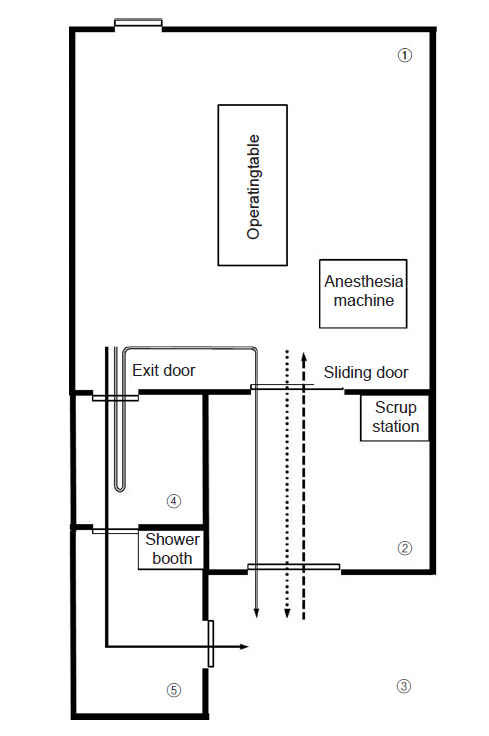
Table 1.
Timeline of Patient with Confirmed SARS-CoV-2
REFERENCES
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239-42.



2. COVID-19 National Emergency Response Center.; Epidemiology and Case Management Team.; Korea Centers for Disease Control and Prevention. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect 2020; 11: 8-14.



3. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth 2020; 67: 568-76.




4. Wong J, Goh QY, Tan Z, Lie SA, Tay YC, Ng SY, et al. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth 2020; 67: 732-45.




5. Tompkins BM, Kerchberger JP. Special article: personal protective equipment for care of pandemic influenza patients: a training workshop for the powered air purifying respirator. Anesth Analg 2010; 111: 933-45.

6. Søreide K, Thorsen K, Harrison EM, Bingener J, Møller MH, Ohene-Yeboah M, et al. Perforated peptic ulcer. Lancet 2015; 386: 1288-98.



7. Ghekiere O, Lesnik A, Hoa D, Laffargue G, Uriot C, Taourel P. Value of computed tomography in the diagnosis of the cause of nontraumatic gastrointestinal tract perforation. J Comput Assist Tomogr 2007; 31: 169-76.


8. Kim JY, Song JY, Yoon YK, Choi SH, Song YG, Kim SR, et al. Middle East respiratory syndrome infection control and prevention guideline for healthcare facilities. Infect Chemother 2015; 47: 278-302.



9. Sehulster L, Chinn RY.; CDC.; HICPAC. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52: 1-42.
10. Park J, Yoo SY, Ko JH, Lee SM, Chung YJ, Lee JH, et al. Infection prevention measures for surgical procedures during a Middle East respiratory syndrome outbreak in a tertiary care hospital in South Korea. Sci Rep 2020; 10: 325.




11. Park MH, Kim HR, Choi DH, Sung JH, Kim JH. Emergency cesarean section in an epidemic of the Middle East respiratory syndrome: a case report. Korean J Anesthesiol 2016; 69: 287-91.




12. Xia H, Zhao S, Wu Z, Luo H, Zhou C, Chen X. Emergency caesarean delivery in a patient with confirmed COVID-19 under spinal anaesthesia. Br J Anaesth 2020; 124: e216-8.



-
METRICS

-
- 3 Crossref
- 5,685 View
- 102 Download
- Related articles in Anesth Pain Med
- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others







