Bleeding properties according to surgical sites during pediatric craniotomy: a retrospective study comparing the two stages of epilepsy surgery
Article information
Abstract
Background
During pediatric epilepsy surgery, due to low circulating blood volume, intraoperative bleeding can result in significant hemodynamic instability, thereby requiring meticulous hemodynamic and transfusion strategies. Knowing the source of bleeding during the procedure would allow medical staff to better prepare the perioperative protocols for these patients. We compared intraoperative bleeding between the first (involving skin to meninges) and second (involving brain parenchyma) stages of epilepsy surgery to investigate the differences between various anatomical sites.
Methods
We reviewed the electronic medical records of 102 pediatric patients < 14 years old who underwent two-stage epilepsy surgeries during January 2012–December 2016. Invasive subdural grids were placed via craniotomy during Stage 1 and the epileptogenic zone was removed during Stage 2 of the surgery. We compared the volume of intraoperative bleeding between these two surgeries and identified variables associated with bleeding using multivariate regression analysis.
Results
Both surgeries resulted in similar intraoperative bleeding (24 vs. 26 ml/kg, P = 0.835), but Stage 2 required greater volumes of blood transfusion than Stage 1 (18.4 vs. 14.8 ml/kg, P = 0.011). Massive bleeding was associated with patients < 7 years of age in Stage 1 and weighing < 18 kg in Stage 2.
Conclusions
The volume of intraoperative bleeding was similar between the two stages of pediatric epilepsy surgery and was large enough to require blood transfusions. Thus, blood loss during pediatric epilepsy surgery occurred at both anatomic sites. This indicates the necessity of early preparation for blood transfusion in both stages of pediatric epilepsy surgery.
INTRODUCTION
Intraoperative bleeding during pediatric craniotomy is challenging for anesthesiologists, with patients often requiring blood transfusions due to their relatively low circulating blood volume [1,2]. Although operation duration and patient age are known risk factors for the requirement of intraoperative blood transfusion during pediatric craniotomy for brain tumor removal [1], there is little information on the main source and volume of intraoperative bleeding. Thus, it is challenging for anesthesiologists to know when and how rheological management strategies should be applied in these patients. Understanding the characteristics of intraoperative bleeding from different anatomical sites during craniotomy would allow medical staff to establish appropriate perioperative strategies.
For drug-resistant epilepsy, which comprises 20–30% of childhood epilepsy cases, patients have the option of one- or two-stage epilepsy surgeries [3–5]. In contrast to one-stage surgery, the main surgical sites for two-stage surgery differ between the first and second stages. The first stage involves the implantation of subdural and/or strip electrodes, and the procedures are mainly performed at the scalp, soft tissue, cranium, and meninges. The second stage is mainly performed at the brain parenchyma and involves removing the epileptic foci or the epileptic transmission pathways [5,6]. Therefore, two-stage epilepsy surgeries could be good candidates for identifying the nature of intraoperative bleeding from different anatomical sites in and around the brain.
In this study, we compared the intraoperative blood loss between the first and second stages of epilepsy surgery to investigate the bleeding properties of different anatomical sites and identify the associated variables during pediatric epilepsy surgeries.
MATERIALS AND METHODS
Ethics approval
This retrospective study was approved by the Institutional Review Board (no. 4-2017-1030) on December 15, 2017. The requirement for written informed consent from patients was waived owing to the retrospective nature of the study.
Patient population
We reviewed the electronic medical records of pediatric patients (younger than 14 years) who underwent only two-stage epilepsy surgeries between January 2012 and December 2016 at the university hospital. Patients were excluded if they underwent any surgical procedures beyond subdural electrode implantation during the first stage or if they underwent any operations due to complications or for other reasons between the two stages.
Anesthesia
Anesthesia was induced with 2 mg/kg propofol (Fresofol 1% MCT, Fresenius Kabi Austria GmbH, Austria), 0.8 mg/kg rocuronium (Rocumeron, Ilsung Pharmaceuticals Co., Ltd., Korea), and 0.5–1 µg/kg remifentanil (Ultian, Hanlim Pharm. Co., Ltd., Korea). Subsequently, a radial artery catheter was inserted, and an intravenous cannula (using a 20-G or larger catheter) was placed in the peripheral or femoral vein. Anesthesia was maintained using sevoflurane in oxygen/air (50%) and remifentanil (0.05–0.15 µg/kg/min). Electrocardiography, pulse oximetry, invasive arterial blood pressure and esophageal temperature measurement, capnography, and gas analysis were performed as standard monitoring. Allogeneic blood products were transfused at the discretion of the neuroanesthesiologist in charge of the patient, based on the patient’s intraoperative hemoglobin level, volume of blood loss, hemodynamic status, and urinary output. After surgery, patients were extubated and transferred to the neurosurgical intensive unit.
Operation
An invasive subdural grid and/or strip electrodes were placed via craniotomy during the Stage 1 surgery. Then, electrocorticography was performed for approximately 7–10 days to identify the epileptic focus or epileptic transmission pathways in preparation of Stage 2 surgery. During Stage 2, the intracranial electrodes were removed, and the epileptogenic zone or pathway of epilepsy transmission was resected.
Variables
Preoperative variables included demographics; anthropometric data; duration and type of antiepileptic drugs (AEDs) received; skull thickness (largest cranial depth at the craniotomy performed at Stage 1, as detected on computed tomography); and laboratory data before and after each surgery, such as hematocrit value, platelet count, coagulation test results (prothrombin time [PT], international normalized ratio [INR]), activated partial thromboplastin time (aPTT), serum calcium level, and levels of other electrolytes (sodium, potassium, chloride, and phosphate). Perioperative variables consisted of the volumes of intraoperative bleeding and blood transfusions, operation duration, and hematocrit value immediately after surgery. The hematocrit level of the arterial blood gas test, conducted after anesthesia and after surgery, was used to calculate the volume of intraoperative bleeding. In Stage 1, total grid area was selected for surgical extent. Total grid area was defined as the sum of the area of the grid and that of the strip electrodes implanted. The surgical type and extent required for Stage 2 were investigated. Loss of more than 50% of the estimated blood volume was defined as massive bleeding [7,8].
Assessment of blood loss
Blood loss (ml) was calculated using the following formulas [9]:
Estimated red cell volume (ERCV)lost = ERCVpreop + ERCVtransfused – ERCVpostop
ERCV = estimated blood volume (EBV) × hematocrit value / 100
The EBV (ml) was 80 ml/kg for infants younger than 12 months and 75 ml/kg for children older than 12 months. The hematocrit value of the transfused packed red blood cells (PRBC) was 60%.
ERCVtransfused = (PRBC [ml])transfused × hematocrit valuetransfused PRBC / 100
The EBV was calculated as follows:
EBVlost (ml/kg) = ERCVlost (ml) / [weight (kg) × hematocrit valuepreop / 100]
Statistical analyses
All values are expressed as mean ± standard deviation, median (1Q, 3Q), or number of patients (%). Normality was tested using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Parametric data were analyzed using the paired t-test (aPTT); nonparametric data were analyzed using the Wilcoxon signed-rank test (hematocrit, calcium, platelet, PT, operation duration, fluid intake, urine output, blood loss, and volume of transfusion). Categorical variables were evaluated using the McNemar’s test (incidence of massive bleeding). The volume of intraoperative bleeding was calculated using the above-mentioned formulas, and the differences between the two stages were analyzed. To identify variables associated with the volume of intraoperative bleeding, a multivariate linear regression model was used. A multivariate logistic regression model was used to identify variables associated with the incidence of massive bleeding. All preoperative variables (sex, age, height, weight, body mass index, body surface area, skull thickness, duration of receiving AEDs, total grid area, hematocrit, calcium, platelet, PT, and aPTT) and operation duration were included in the regression analysis. The final linear and logistic regression models were determined using backward selection among significant variables in the multivariate regression with the enter method. In this analysis, the variables of height, body mass index, and body surface area, which were strongly related to the weight of the pediatric patients, were excluded to reduce multicollinearity. The optimal cut-off value of the logistic regression analysis was calculated using the Youden index [10]. The predictive performance of the final model was quantified by the area under the curve with a 95% confidence interval. The Hosmer–Lemeshow test was used to assess goodness of fit for the logistic regression model. Correlation analyses were performed using the Pearson or Spearman methods. P value < 0.05 was considered to be statistically significant. All analyses were performed using SPSS version 23.0 (IBM Corp., USA) and MedCalc Statistical Software version 18.11.3 (MedCalc Software bvba, Belgium).
RESULTS
During the study period, 109 pediatric patients (younger than 14 years) underwent two-stage epilepsy surgeries (Fig. 1). Of these, four and three patients underwent subdural and epidural hematoma removal, respectively, between Stage 1 and 2. These patients were excluded, leaving a total of 102 pediatric patients (median age, 7.3 years) to be studied. Preoperative patient characteristics are presented in Table 1. The Stage 2 surgeries included frontal, temporal, parietal, or/and occipital lobectomy; amygdalectomy; callosotomy; or/and lesionectomy. The total grid area (mm2) in Stage 1 was 112 (80, 128). Preoperative laboratory values and operative data are presented in Table 2. None of the patients reported elevated PT/aPTT or took anticoagulants.
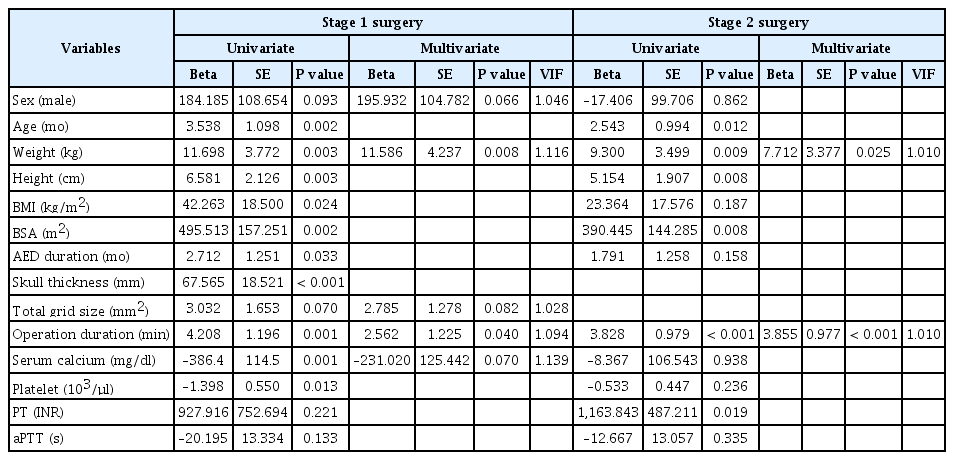
There were no statistical differences in the volume of intraoperative bleeding (24 vs. 26 ml/kg, P = 0.835) or in the incidence of massive bleeding (27% vs. 32%, P = 0.516) between the two surgery stages. Preoperative hematocrit values (%) and serum calcium levels (mg/dl) were lower in Stage 2 than in Stage 1 (30.5 vs. 33.9, P < 0.001; 8.8 vs. 9.3, P < 0.001). Preoperative PT (INR) was prolonged in Stage 2 compared to that in Stage 1 (1.07 vs. 0.98, P < 0.001). The volume of blood transfusion (ml/kg) was higher in Stage 2 than in Stage 1 (18.4 vs. 14.8, P = 0.011). None of the patients received fresh frozen plasma or platelet transfusions. Multivariate linear regression analysis showed that patient weight and operation duration were associated with blood loss during both surgery stages (Table 3).
The variables associated with massive bleeding in Stage 1 were age (cut-off value of 7.5 years) and PT (INR, cut-off value of 0.96); in Stage 2, the associated variables were weight (cut-off value of 18.1 kg) and PT (INR, cut-off value of 1.12) (Table 4). Receiver operating characteristics curve analysis of the aforementioned regression models predicted intraoperative massive bleeding with an area under the curve of 0.803 (95% confidence interval, 0.712–0.875) during Stage 1 and 0.778 (95% confidence interval, 0.682–0.857) during Stage 2.
The duration of receiving AEDs was not correlated with preoperative PT or aPTT (P = 0.335 and P = 0.962, respectively).
DISCUSSION
For pediatric patients undergoing craniotomy, careful planning of hemodynamic management and blood transfusion strategies is required to avoid hypovolemia. In this study, we found that the volume of intraoperative bleeding was comparable between the two stages (i.e., Stage 1 vs. 2, involving the structures from the scalp to meninges vs. the brain parenchyma) of pediatric epilepsy surgery; however, Stage 2 required more blood transfusions than Stage 1. Massive bleeding during the surgery was associated with low body weight or young age and elevated PT.
Timely preparation with adequate red blood cell volumes has been challenging for anesthesiologists in the management of pediatric craniotomy. The study by Schmotzer et al. [11] regarding preoperative red blood cell preparation and utilization among pediatric surgical procedures revealed that the preparations of red blood cells for such procedures were much higher than its actual usage for transfusions. Interestingly, in their study, neurosurgery had a relatively lower ratio of red cell preparation to transfusion than other surgeries because intraoperative transfusion events were highest in neurosurgery, including epilepsy surgeries. However, Schmotzer et al. [11] did not mention whether the epilepsy surgeries were one- or two-stage surgeries; thus, the information regarding the anatomical source of bleeding or of the optimal timing for rheological management strategies in pediatric epilepsy surgeries could not be obtained. In our study, we observed that the Stage 1 surgery can induce significant intraoperative bleeding, similar to levels seen in Stage 2. The significance of our finding was highly evident for massive bleeding; patient age < 7 years in Stage 1 and body weight < 18.1 kg in Stage 2 were significant variables in addition to prolonged PT. Thus, blood loss during pediatric craniotomy occurred at both anatomical sites, from the skin to meninges as well as from the brain parenchyma. Therefore, we recommend early preparation of red blood cells for transfusion in patients who are less than 7 years of age or weighing less than 18 kg.
Regarding the transfusion of red blood cells, we found that Stage 2 required larger volumes of blood transfusion than Stage 1, despite similar volumes of intraoperative bleeding. This difference in the volume of blood transfusion between the two surgeries could be attributed to lower hematocrit values and serum calcium levels and to prolonged PT (INR) and operation durations associated with Stage 2 surgery. It is expected that young patients (< 7 years) undergoing craniotomy could be vulnerable to hemodynamic instability before surgical manipulation at the brain parenchyma begins. In a previous study, brain tumor removal in children required 16 ml/kg of allogenic transfusion [1]. This is comparable to the amount of allogenic transfusion required in epilepsy surgeries (14.8 and 18.4 ml/kg in the first and second stages of epilepsy surgery in our study, respectively), despite the difference in the type of surgery, underlying pathology, age distribution, etc. [1]. Considering the requirement for a larger volume of blood transfusion during two-stage epilepsy surgeries, a special strategy—similar to that for pediatric cardiac surgery [12,13]—may be needed to enhance hematopoiesis using iron replacement or recombinant erythropoietin or to minimize intraoperative bleeding using antifibrinolytic agents [14,15].
As a second purpose of this study, we looked for the variables associated with volume of intraoperative bleeding in the first and second stages of surgeries. The duration of receiving AEDs and the skull thickness were statistically significant for Stage 1 only in the univariate analysis, but they were insignificant in the multivariate analysis. Indeed, it is uncertain whether AEDs thicken the skull and increase bleeding tendency due to their effects on bone metabolism, bone matrix calcium, and platelet fraction [16,17]. Pediatric patients taking AEDs such as carbamazepine, phenytoin, and valproic acid have been shown to exhibit thrombocytopenia and severe intraoperative bleeding [18–20]. In addition, valproic acid and gabapentin are associated with acquired von Willebrand disease type 1, hypofibrinogenemia, decreased factor XIII, and abnormal platelet function [21–23]. However, Manohar et al. [24] failed to demonstrate the effects of AEDs on perioperative blood loss or coagulopathy. Besides this, the serum calcium level is a key cofactor in the coagulation cascade [25,26]. A low serum calcium level (< 8.4 mg/dl) correlates with increased bleeding and higher 30-day mortality rate in patients with intracranial hemorrhage compared to those in patients with normal serum calcium levels [27]. In the present study, the serum calcium level was associated with Stage 1 intraoperative bleeding only in the univariate analysis, but not in the multivariate analysis, and was not associated with intraoperative and massive bleeding during Stage 2. In the multivariate regression analysis, the variables related to the volume of intraoperative bleeding in the two-stage epilepsy surgeries were the operation duration and patient body weight, which were comparable to the results reported previously [1].
Some limitations of our study should be acknowledged. First, this retrospective observational study was conducted at a single institute. Therefore, our results might not be applicable to patients from other institutes, given that surgical variables differ among different institutes and medical environments. Second, we were not able to control the extent of surgery in this study, despite having measured total grid size for the size of surgical extent in Stage 1. During Stage 2, the operation sites were too diverse to categorize and analyze. Other surgical variables, such as procedural proximity to vascularity at the surgical site, could be associated with intraoperative bleeding during craniotomy for epilepsy surgery, regardless of the stage. We could not measure these additional variables owing to the retrospective nature of the study. Third, participants were recruited over a long period of time, during which the AED regimen may have changed. Fourth, the Stage 2 surgeries were performed 7–10 days after Stage 1, and the status of red cell mass as well as coagulation profiles during Stage 2 were likely affected by the immediate postoperative status of Stage 1.
In conclusion, the volume of intraoperative bleeding in the first and second stages of two-stage epilepsy surgeries was similar and significant enough to require blood transfusion. This indicates that the anatomical sites of intraoperative bleeding during pediatric epilepsy surgery were both from the skin to the meninges as well as the brain parenchyma. Therefore, early preparation for blood transfusion is needed in pediatric patients undergoing the two stages of epilepsy surgery, especially in patients who are < 7 years of age or weighing < 18 kg.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: Bora Lee, Kyeong Tae Min. Data acquisition: Myung Il Bae, Darhae Eum, Albel Mussa Ntungi, Byongnam Jun. Formal analysis: Bora Lee. Supervision: Kyeong Tae Min. Writing—original draft: Bora Lee. Writing—review & editing: Bora Lee, Kyeong Tae Min.