 |
 |
- Search
| Anesth Pain Med > Volume 19(1); 2024 > Article |
|
Abstract
Background
Systolic murmur suggesting the association of aortic valve (AV) stenosis or obstructive pathology in the left ventricular outflow tract (LVOT) usually requires preoperative echocardiographic evaluation for elective surgery.
Case
In a 63-year-old female patient undergoing elective thoracic surgery, the systolic murmur was auscultated on the right sternal border of the second intercostal space in the preoperative patient holding area. Point-of-care (POC) transthoracic echocardiography (TTE) demonstrated a systolic jet flow in the LVOT area. The peak systolic velocity of the continuous wave Doppler tracing, aligned to the LVOT and the AV, was approximately 1.5 m/s. The peak/mean pressure gradient was 11/6 mmHg for the AV and 9/5 mmHg for the LVOT. Anesthesia was induced under continuous TTE imaging. Intraoperative transesophageal echocardiography also confirmed the absence of any cardiac pathology.
Point-of-care (POC) echocardiography is helpful in critical decision-making, but its efficacy can be limited due to the simplified capability of hand-held echocardiography devices. In the present case, POC echocardiography provided a quick and thorough cardiac evaluation of a sudden systolic murmur and prompted planned elective surgery by avoiding conventional preoperative cardiac evaluation.
A 63-year-old female with a 15-year history of well-controlled hypertension (height of 152.9 cm and weight of 74.5 kg) was scheduled for a video-assisted thoracic surgery for a slowly growing pulmonary nodule in the right upper lobe (RUL). While no cardiac symptoms had been noted during the routine pre-anesthetic evaluation, an intermittent systolic murmur was pointed out on the patient’s arrival at the patient-holding area. The murmur was pan-systolic in a conventional stethoscope and audible at the right sternal border of the second intercostal space in the supine position. Position change and the Valsalva maneuver did not affect the characteristics of the murmur. It was necessary to identify aortic valve (AV) stenosis, as well as to identify hypertrophic cardiomyopathy that could cause left ventricular outflow tract (LVOT) obstruction.
An attending anesthesiologist performed POC 2-dimensional (2D) transthoracic echocardiography (TTE, Epiq CVX™ cardiovascular ultrasound system and X5-1™ 3-dimensional [3D] transducer, Philips) to evaluate whether the systolic murmur had a potential association with severe AV stenosis or LVOT obstruction (Fig. 1A, Video 1A). The AV area was estimated as 2.11-2.33 cm2 and the LVOT was as 1.79-1.98 cm2, respectively, by using the continuity equation. The peak/mean pressure gradient was 11/6 mmHg for the AV and 9/5 mmHg for the LVOT (figures were not presented).
In apical 4-chamber imaging, the left ventricle (LV) wall motion and Doppler analyses (mitral valve [MV] inflow and MV annular motion) suggested well-preserved systolic and diastolic performance: LV ejection fraction of 62%; the ratio of early diastolic MV inflow to early diastolic MV annulus motion (E/e' ratio) was 4.5; and the LV 4-chamber longitudinal strain and right ventricular free-wall longitudinal strain, measured by on-cart software (Q-Lab™, Philips), were -23.2% and -26.1%, respectively (Fig. 1B, Video 1B).
The peak systolic velocity of the continuous wave Doppler tracing, aligned to the LVOT and AV in a modified apical 4-chamber view, was approximately 1.5 m/s and unaffected by applying the Valsalva maneuver (Fig. 1C).
The parasternal long-axis TTE image provided detailed information on the LVOT flow around the AV, MV apparatus, hypertrophied LV septum, and LVOT: no apparent pathology suggesting AV stenosis, such as calcification, fusion, or limited motion of AV leaflets, was noted (Fig. 1D, Video 1C). Despite systolic jet flow in the LVOT area, the geometry of the MV apparatus and the LVOT did not have risk factors for potentially developing systolic anterior motion (SAM) of the anterior MV leaflet or dynamic LVOT obstruction: the distance between the MV coaptation and the hypertrophied LV septum (C-Sept) was far greater than 25 mm, and the ratio of the length of the anterior to posterior MV leaflets was far greater than 1.5 [1].
The planned lobectomy proceeded without delay for performing a conventional preoperative echocardiographic evaluation. Two anesthesiologists induced general anesthesia under continuous monitoring of invasive-arterial pressure and parasternal TTE imaging.
After anesthesia induction, real-time 3D transesophageal echocardiography (TEE, X8-2t xMatrix™ 3D transducer, Philips) confirmed the POC TTE findings. It provided much more detailed information about the origin and the nature of the murmur: the systolic jet flow arose from the narrow space surrounded by the anterior MV chordae tendineae (at the tip of the posteromedial papillary muscle) and the hypertrophied inferior LV wall (Fig. 2A and B, Video 2A and B). The jet traversed the LVOT area and reached the AV annulus, and its flow intensity was variable to the changes in the LV filing status (Fig. 2C, D).
The planned RUL lobectomy was completed, and the patient was discharged without any clinical event with hemodynamic instability. Before the discharge, a cardiologist performed a TTE due to an instantaneous aggravation of the murmur and concluded that the murmur was physiologic and required no further treatment.
Informed consent for publication of the present case was obtained from the patient.
The present case showed the value of POC TTE in providing an immediate cardiac evaluation in a patient presenting a sudden systolic murmur. POC TTE enabled the quick assessment of the systolic murmur and ruled out the current and potential association of severe AV stenosis or dynamic LVOT obstruction. The immediate and comprehensive cardiac evaluation prompted elective surgery without case cancellation for further echocardiographic evaluation.
Focused cardiac ultrasound performed by non-cardiologists (FOCUS, a similar definition of POC echocardiography) has provided essential information regarding associated cardiac pathologies and facilitated clinical decision-making in various critical scenarios [2]. FOCUS performed by anesthesiologists, especially during the preoperative period, can add enormous benefit to gaining insight regarding the associated pathologies, facilitating time-sensitive clinical decision-making in the surgical arena, and improving perioperative management [3,4].
Systolic murmur is common in old ages [5,6], but its association suggests a higher likelihood of AV stenosis or obstructive pathology in LVOT. Therefore, proceeding or postponing the elective surgical case without further evaluation can be challenging in patients presenting systolic murmur. As in the present case, the dynamic nature of the murmur, as indicated by its absence of consistency, may complicate the complexity. Since perioperative anesthesia-induced vasodilation is critical to patients with severe AV stenosis and insufficiently attenuated noxious stimuli can induce catastrophic hemodynamic collapse in patients with dynamic LVOT obstruction, it is necessary to implement intense perioperative hemodynamic vigilance and thorough preoperative POC cardiac evaluation in a patient suddenly presenting a systolic murmur.
The peak velocity of the continuous-wave Doppler tracing aligned to the LVOT and AV was approximately 1.5 m/s (pressure gradient reaching about 9 mmHg) and not affected by applying the Valsalva maneuver. The peak pressure gradient and the discrete nature of the flow velocity enabled the exclusion of the possible association between AV stenosis and LVOT obstruction.
In the present case, in addition to the invasive BP monitoring, POC TTE monitoring was applied to detect and avoid dynamic LVOT obstruction due to relative hypovolemia and increased Venturi effect aggravating the risks during anesthesia induction [7,8]. In addition, the papillary muscle and the LV inferior wall. The jet's size and intensity were changing specifically to the LV filling status and contractility.
Meanwhile, the Venturi effect (sucking) is not the only factor developing SAM and dynamic LVOT obstruction. Unfavorable geometry of MV leaflet apparatus and LVOT reducing C-Sept, such as hypertrophied LV septum and a relatively longer posterior MV leaflet (compared to anterior MV leaflet) [1,9], can increase the risk of dynamic LVOT obstruction. In patients with shorter C-Sept, systolic LV flow can easily drag the anterior MV leaflet into LVOT [1,8,9].
In the present case, C-Sept, far longer than 25 mm, could exclude the potential risk of dynamic LVOT obstruction, even in unexpected LV filling status or intrathoracic pressure changes [1]. The variable intensity of the jet flow in 2D and 3D TTE and TEE imaging, specific to the changes in the LV dimension and filling status, may explain the inconsistency of the systolic murmur: preoperative fasting and dehydration might reduce LV dimension and exaggerate the systolic jet and murmur, which could be apparent in auscultation immediately before the start of the case [10,11].
In the meantime, there is no consensus regarding the extent of POC TTE. In most cases, the coverage may be limited to evaluating primary structural and functional cardiac problems because of the limited capabilities of hand-held devices. POC TTE can roughly detect SAM of anterior MV leaflet and dynamic LVOT obstruction, in addition to confirming the presence of valvular stenosis or regurgitation using Doppler [12]. A simple hand-held ultrasound device may be sufficient for POC TTE examination to assess murmurs, hemodynamic instability, ventricular function, and etiology of dyspnea [13]. In surgeries like hip fractures that are not emergencies but are essential, proceeding with the procedure can reduce the overall mortality rate [14] and prevent delays in treatment when necessary [15]. As in the present case, if a sophisticated and high-end TTE device (4-dimensional echo probe with sophisticated on-cart software analyzing LV wall motion, such as strain and tissue Doppler) can be applied by experienced anesthesiologists, the scope of POC TTE can be expanded to the thorough assessment of the valvular flow Doppler and cardiac wall motion.
Notes
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Jaemoon Lee. Writing - review & editing: Minki Lee, Sookyung Lee, Chung-Sik Oh, Tae-Yop Kim. Conceptualization: Jaemoon Lee, Minki Lee. Data curation: Jaemoon Lee, Minki Lee, Sookyung Lee, Chung-Sik Oh. Project administration: Jaemoon Lee, Minki Lee, Sookyung Lee, Chung-Sik Oh. Resources: Minki Lee. Supervision: Tae-Yop Kim.
SUPPLEMENTARY MATERIALS
Supplementary video is available at https://doi.org/10.17085/apm.23124.
Video 1.
Preoperative 2-dimensional TTE imaging. (A) Apical 4-chamber view with poor nearfield delineation of LV wall motion. (B) Global longitudinal strain of LV and RV in apical 4-chamber view was -23.2% and -26.1%, respectively. (C) The distance between the MV coaptation and LV septum (C-Sept) is far greater than 2.5 cm in the parasternal long-axis view (two white dotted lines indicates the anterior and posterior MV leaflets). The systolic jet flow in the LVOT area is noted in the parasternal long-axis view (arrow). Ao: aortic valve opening, AV: aortic valve, C-sept: the distance between the mitral valve coaptation and left ventricular septum (yellow dotted line with reversed arrows in both ends), LA: left atrium, LV: left ventricular cavity, LV GLS A4C: LV global longitudinal strain in apical four-chamber view, LVOT: LV outflow tract, MV: mitral valve, RA: right atrium, RV: right ventricle, RV FWS: RV free wall strain, SAM: systolic anterior motion, TTE: transthoracic echocardiography.
Video 2.
Intraoperative 2D and 3D TEE imaging. (A) modified 2D deep transgastric long-axis view with color Doppler delineates the systolic jet flow (arrow) arising from the tip of anterolateral papillary muscle and anterior LV wall, traversing LVOT area and ending just before the AV opening. (B) 3D mid-esophageal TrueVueTM image visualizes the narrow structure in the LV cavity where the jet flow arises (arrow). (C) Multi-planar images, reconstructed from a 3D volume color data, delineates the long-axis tracing (direction) of the jet flow, the origin of the jet flow (a narrow space between the tip of the posteromedial papillary muscle and the LV inferior wall in the left upper green plane and right upper red plane), and the short-axis tracing of the jet origin (vena contracta, in the left lower blue plane). Ao: aortic valve opening, AV: aortic valve, LV: left ventricular cavity, LVOT: LV outflow tract, LVw: LV anterior wall, P: anterolateral papillary muscle, TEE: transesophageal echocardiography, 2D: 2-dimensional, 3D: 3-dimensional.
Fig. 1.
Preoperative TTE imaging. (A) Apical 4-chamber view with poor nearfield delineation of LV wall motion. (B) Global longitudinal strain of LV and RV in apical 4-chamber view was -23.2% and -26.1%, respectively. (C) Continuous-wave Doppler tracing of LVOT and AV blood flows in the modified apical 4-chamber view is approximately 1.5 m/sec. (D) The distance between the MV coaptation and LV septum (C-Sept) is far greater than 2.5 cm in the parasternal long-axis view (two white dotted lines indicates the anterior and posterior MV leaflets). (E) In the absence of AV stenosis, SAM of anterior MV leaflet or LVOT obstruction, the systolic jet flow in the LVOT area is noted in the parasternal long-axis view. Ao: aortic valve opening, AV: aortic valve, C-sept: the distance between the mitral valve coaptation and left ventricular septum (yellow dotted line with reversed arrows in both ends), LA: left atrium, LV: left ventricular cavity, LV GLS A4C: LV global longitudinal strain in apical four-chamber view, LVOT: LV outflow tract, MV: mitral valve, RA: right atrium, RV: right ventricle, RV FWS: RV free wall strain, SAM: systolic anterior motion, TTE: transthoracic echocardiography.
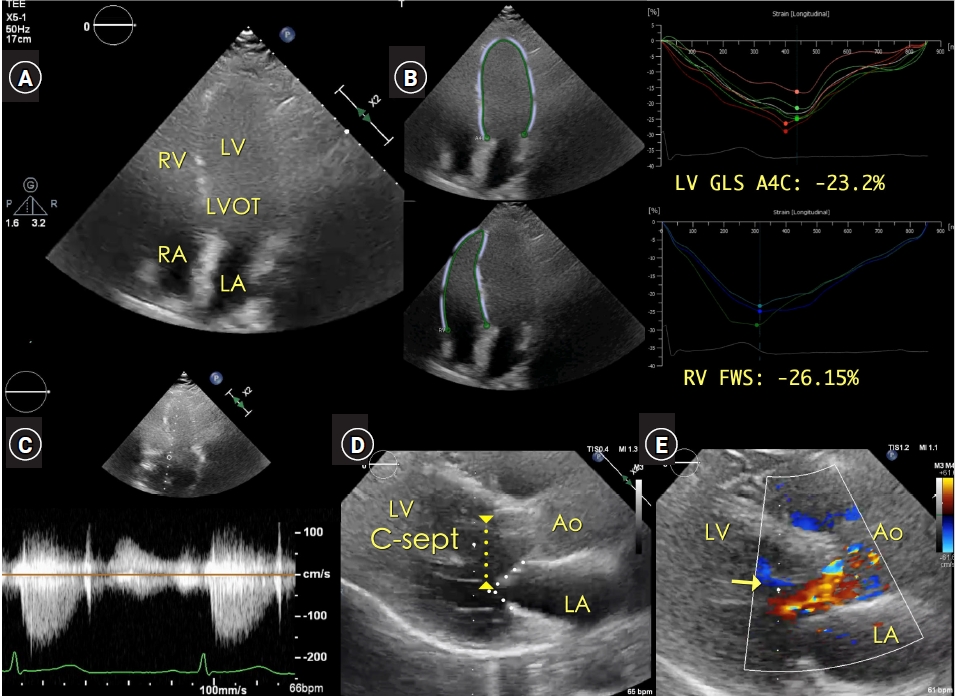
Fig. 2.
Intraoperative TEE imaging. (A) Modified 2D deep transgastric long-axis view with color Doppler delineates the systolic jet flow (arrow) arising from the tip of anterolateral papillary muscle and anterior LV wall, traversing LVOT area and ending just before the AV opening. (B) 3D mid-esophageal TrueVue™ image visualizes the narrow structure in the LV cavity where the jet flow arises (arrow). (C) Multi-planar images, reconstructed from a 3D volume color data, delineates the long-axis tracing (direction) of the jet flow, the origin of the jet flow (a narrow space between the tip of the posteromedial papillary muscle and the LV inferior wall in the left upper green plane and right upper red plane), and the short-axis tracing of the jet origin (vena contracta, in the left lower blue plane). (D) Rotated 3D TrueVue™ live image localizes the origin of the jet flow (arrow) with adjacent cardiac structures. The size of the jet flow color Doppler was variable to the changes in LV filling status and contractility. Ao: aortic valve opening, AV: aortic valve, LV: left ventricular cavity, LVw: LV anterior wall, LVOT: LV outflow tract, P: anterolateral papillary muscle, TEE: transesophageal echocardiography, 2D: 2-dimensional, 3D: 3-dimensional.

REFERENCES
1. Maslow AD, Regan MM, Haering JM, Johnson RG, Levine RA. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol 1999; 34: 2096-104.


2. Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr 2014; 27: 683.e1-e33.


3. Kalagara H, Coker B, Gerstein NS, Kukreja P, Deriy L, Pierce A, et al. Point-of-Care Ultrasound (POCUS) for the cardiothoracic anesthesiologist. J Cardiothorac Vasc Anesth 2022; 36: 1132-47.


4. Ramsingh D, Bronshteyn YS, Haskins S, Zimmerman J. Perioperative Point-of-Care Ultrasound: from concept to application. Anesthesiology 2020; 132: 908-16.

5. Roelandt JRTC. The decline of our physical examination skills: is echocardiography to blame? Eur Heart J Cardiovasc Imaging 2014; 15: 249-52.


6. Loxdale SJ, Sneyd JR, Donovan A, Werrett G, Viira DJ. The role of routine pre-operative bedside echocardiography in detecting aortic stenosis in patients with a hip fracture. Anaesthesia 2012; 67: 51-4.



7. Jiang L, Levine RA, King ME, Weyman AE. An integrated mechanism for systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy based on echocardiographic observations. Am Heart J 1987; 113: 633-44.


8. Levine RA, Vlahakes GJ, Lefebvre X, Guerrero JL, Cape EG, Yoganathan AP, et al. Papillary muscle displacement causes systolic anterior motion of the mitral valve. Experimental validation and insights into the mechanism of subaortic obstruction. Circulation 1995; 91: 1189-95.


9. Klues HG, Roberts WC, Maron BJ. Morphological determinants of echocardiographic patterns of mitral valve systolic anterior motion in obstructive hypertrophic cardiomyopathy. Circulation 1993; 87: 1570-9.


10. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J 2023; 44: 3503-626.

11. Houston BA, Stevens GR. Hypertrophic cardiomyopathy: a review. Clin Med Insights Cardiol 2015; 8(Suppl 1): 53-65.




12. Barber RL, Fletcher SN. A review of echocardiography in anaesthetic and peri-operative practice. Part 1: impact and utility. Anaesthesia 2014; 69: 764-76.


13. Halvorsen S, Mehilli J, Cassese S, Hall TS, Abdelhamid M, Barbato E, et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J 2022; 43: 3826-924.

- TOOLS
- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others








