INTRODUCTION
Neuromuscular blocking agents are frequently used to facilitate endotracheal intubation during the induction of anesthesia [
1]. However, neuromuscular blockers are associated with prolonged neuromuscular blockade, histamine release, and anticholinesterase-mediated side effects [
2-
5]. Furthermore, the use of neuromuscular blockers is either avoided or contraindicated in some patients. Therefore administration of a proper induction agent and adjuvant to provide good intubating conditions without the use of a neuromuscular blocker may be an important consideration [
2-
4].
Propofol and remifentanil (2-4 μg/kg) are known to be able to induce a condition appropriate for endotracheal intubation without a neuromuscular blocking agent [
6]. According to a previous study, the effective doses of remifentanil in 50% (ED
50) and 95% (ED
95) of patients were 1.4 μg/kg and 2.4 μg/kg, respectively, during induction of anesthesia with propofol 2 mg/kg without a neuromuscular blocker [
7]. Remifentanil may improve the intubating conditions in a dose-dependent manner, but adverse effects such as hypotension, bradycardia, and muscular stiffness may occur as the dose is increased [
6]. If administered with propofol, remifentanil may cause hypotension and bradycardia requiring rescue medication in 50% of patients who receive doses as low as 1 μg/kg [
8]. Moreover, bolus injection of a large dose of remifentanil may result in muscle rigidity, which makes mask ventilation difficult [
6]. Thus, doses of over 2 μg/kg of remifentanil, although known to be able to induce a relatively appropriate condition for endotracheal intubation without a neuromuscular blocker, also result in unavoidable adverse effects [
6].
Dexmedetomidine, a highly selective α2-adrenoreceptor agonist, has good analgesic and sedative effects [
9,
10] to decrease the maximum alveolar concentration (MAC) of inhalational anesthetics; for example, it can decrease the MAC of sevoflurane by 17% [
11] and decrease the demand for propofol and opioid in total intravenous anesthesia [
12,
13]. In addition, when used as an adjuvant for induction of anesthesia, dexmedetomidine improves hemodynamic stability by decreasing the hemodynamic changes caused by endotracheal intubation [
14]. Furthermore, dexmedetomidine is known to have sedative and airway reflex blunting effects, providing better conditions for awake fiberoptic intubation, awake blind nasotracheal intubation, and laryngeal mask airway placement when used with propofol [
15-
17].
Thus, when performing endotracheal intubation without a neuromuscular blocker, dexmedetomidine may provide good intubating conditions without the use of a relatively high dose of remifentanil.
In the present study, we investigated the effect of 1 μg/kg dexmedetomidine on the intubating conditions and hemodynamic changes during endotracheal intubation following anesthetic induction performed using propofol 2 mg/kg and remifentanil 1.5 μg/kg without a neuromuscular blocking agent.
MATERIALS AND METHODS
The subjects of the present study were adult patients aged between 20 and 65 years, classified as American Society of Anesthesiologists Physical Status 1 or 2, who were scheduled to undergo an operation under general anesthesia. Patients with a history of sensitization to dexmedetomidine, hypertension, left ventricular ejection fraction of 40 or lower, ischemic heart disease, asthma, respiratory diseases, liver or renal function abnormalities, history of heavy alcohol consumption, or history of chronic hypnotic or analgesic use were excluded. Patients whose physical characteristics suggested difficulties in intubation (modified Mallampati score III or IV) and those who had a previously documented failed intubation were also excluded. The study was approved by the Hospital Ethics Committee, and written informed consent was obtained.
All patients were administered midazolam 2 mg and glycopyrrolate 0.2 mg by intramuscular injection and famotidine 20 mg by intravenous injection as premedication 30 minutes before arrival in the operating room. The noninvasive blood pressure, electrocardiogram, peripheral pulse oxygen saturation, end-tidal CO2, and bispectral index (BIS) measurements of the patients were obtained with standard monitoring. Group D received 1 μg/kg dexmedetomidine (100 μg/ml; Precedex, Hospira Inc., USA) diluted to a total volume of 10 ml and infused over 10 minutes using a calibrated electronic infusion pump (Braun Infusomat, Braun, Germany). Group C received saline solution of equal volume to that of Group D. Preoxygenation was performed for 3 minutes with 5 L/min of 100% oxygen. Anesthesia was induced in an identical manner in both groups using 2 mg/kg propofol and an IV bolus dose of remifentanil 1.5 μg/kg. Tracheal intubation was performed 1 minute after the administration of remifentanil. After consciousness and the lid reflex were lost and the BIS value dropped below 50, endotracheal intubation was performed using a No. 7.5 endotracheal tube for male patients and a No. 7 tube for female patients. For all patients, intubation was performed by the same anesthesiologist, who had not been informed of the drug being used.
The drugs used in the experiments were prepared by nurses of the Department of Anesthesiology and Pain Medicine who did not participate in the present study. A double-blind method was maintained by ensuring that the doctors who were in charge of the anesthesia, the assessment of the endotracheal intubating conditions, and the measurement of vital signs were not cognizant of the study plan or the drugs used.
Based on the criteria developed by Cooper et al. [
18] for assessing intubating conditions, the ease of laryngoscopy, the condition of the vocal cords, and the response to endotracheal intubation were evaluated on a four-point scale (0-3), and the total scores were added together to give an overall intubation score for each patient (Tables
1 and
2). A score of 8-9 was considered excellent, 6-7 good, 3-5 poor, and 0-2 bad; scores of good and excellent were considered clinically acceptable. Poor and bad scores were defined as failed intubations. Patients with poor or bad conditions underwent intubation after the administration of rocuronium 0.6 mg/kg. Patients who received rocuronium to facilitate intubation were included in the bad or poor class and were analyzed for every measurement performed in the present study.
Table 1
Assessment of Intubating Conditions
|
Score |
Laryngoscopy |
Condition of vocal cords |
Response to intubation |
|
0 |
Poor (impossible) |
Closed |
Severe coughing or bucking |
|
1 |
Minimal (difficult) |
Closing |
Mild coughing |
|
2 |
Moderate (fair) |
Moving |
Slight diaphragmatic movement |
|
3 |
Good (easy) |
Open |
None |
Table 2
Intubating Conditions Classification
|
Points |
8-9 |
6-7 |
3-5 |
0-2 |
|
Intubating conditions |
Excellent |
Good |
Poor |
Bad |
After confirmation of a successful endotracheal intubation, the balloon was carefully inflated until the moment when the leaking sound was no longer heard at the neck and the mouth. The tubing position was fixed with bilateral auscultation of pulmonary sounds. Following the endotracheal intubation, anesthesia was maintained using sevoflurane 1.5-2 vol%, and an oxygen:air mixture (2 L/min:2 L/min). The systolic blood pressure, diastolic blood pressure, mean arterial blood pressure, and heart rate were recorded at baseline, 10 minutes after the administration of dexmedetomidine, immediately after the induction of anesthesia, immediately after the endotracheal intubation, 3 minutes after the intubation, and 5 minutes after the intubation.
Atropine 0.5 mg was prepared for intravenous injection if the heart rate decreased to 45 beats/min or lower, and ephedrine 5 mg was prepared for intravenous injection if the systolic blood pressure decreased to 80 mmHg or lower. Hypertension (≥ 20% baseline for > 1 minute) and tachycardia (≥ 20% baseline for > 1 minute) were managed by injection of nicardipine (2-10 μg/kg/min) or labetalol 5-10 mg.
Based on the assumption of an 80% possibility that the use of dexmedetomidine may improve the endotracheal intubation condition to an excellent level, the number of samples was determined using the Epicalc package of the R statistical software (ver. 3.0.0, The R Foundation for Statistical Computing, Vienna, Austria; Chi-square, effect size 0.35). The alpha error was determined as 0.05, and the power as 0.8 for the calculation. When the same number of samples were allocated to each group, 35 samples were required for each group.
The statistical analysis was performed by using the R software (ver. 3.0.2, The R Foundation for Statistical Computing, Vienna, Austria) for Windows. A Shapiro-Wilk test was performed as the normality test if the data were continuous variables. A parametric test was performed if the data showed a normal distribution. For the parametric test, a test for equality of variance was performed with respect to the two groups. If a group satisfied equality of variance, a two-sample t-test was performed. Otherwise, Welch’s test was performed. When a variable did not show a normal distribution, a non-parametric test was performed. If there was not a tie value, the Wilcoxon rank sum test was performed or, if there were more than two groups, the Kruskal-Wallis test was performed. If there was a tie value, Yuen`s test was performed to compare the 20% trimmed mean. Comparison of the hemodynamic changes between the two groups was tested by performing analysis of covariance (ANCOVA). The significance level (P value) was set at 0.05.
RESULTS
All enrolled patients were included in the analysis. There were no significant differences among age, weight, height, and sex between the two groups (
Table 3).
Table 3
|
Group C (n = 35) |
Group D (n = 35) |
|
Female/Male |
24/11 |
27-Aug |
|
Age (yr) |
44.7 ± 8.7 |
39.1 ± 11.7 |
|
Weight (kg) |
61.9 ± 10.3 |
59.5 ± 10.8 |
|
Height (cm) |
159.2 ± 5.5 |
160.2 ± 11.8 |
In the classification of endotracheal intubating conditions according to the endotracheal intubation score, the condition was excellent in 4 patients, good in 20 patients, poor in 4 patients, and bad in 7 patients in Group C, while the condition was excellent in 34 patients and good in 1 patient in Group D (P < 0.001), indicating that the endotracheal intubating conditions were significantly better in the group in which dexmedetomidine was used (
Table 4). The endotracheal intubation failed in 11 patients in Group C, whereas the endotracheal intubation was successful in all patients in Group D.
Table 4
Intubating Conditions Classification
|
Group C (n = 35) |
Group D (n = 35) |
|
Excellent |
4 |
34 |
|
Good |
20 |
1 |
|
Poor |
4 |
0 |
|
Bad |
7 |
0 |
Hemodynamic changes for Groups C and D are shown in Figs.
1 and
2. For Group D, compared to the baseline value, significant decreases in heart rate were observed at all measurement times. The heart rate was significantly lower in Group D than in Group C at 10 minutes after dexmedetomidine administration (P < 0.001), immediately after the induction of anesthesia (P = 0.007), immediately after endotracheal intubation (P = 0.007), at 3 minutes after endotracheal intubation (P = 0.002), and at 5 minutes after endotracheal intubation (P = 0.014), indicating that there were significant differences between the groups at all measurement times (
Fig. 1).
Fig. 1
The changes in mean heart rate (HR) from initiation of dexmedetomidine or saline infusion to 5 min after endotracheal intubation. Error bar means standard error. ***P < 0.001, **P < 0.01, *P < 0.05 vs. Group C, †††P < 0.001, ††P < 0.01, †P < 0.05 vs. baseline. Group C: The group injected with 0.9% normal saline. Group D: The group injected with dexmedetomidine 1 μg/kg. HR: heart rate.
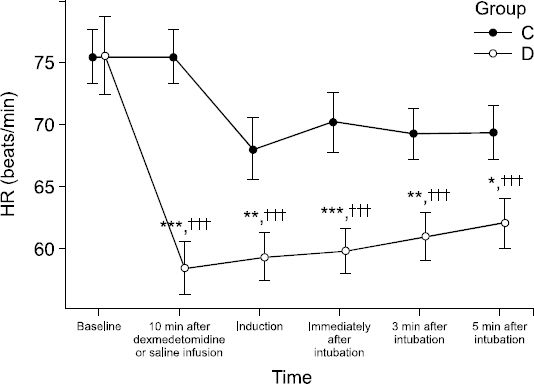
Fig. 2
The changes in mean blood pressure (MBP) from initiation of dexmedetomidine or saline infusion to 5 min after endotracheal intubation. Error bar means standard error. ***P < 0.001, **P < 0.01, *P < 0.05 vs Group C, †††P < 0.001, ††P < 0.01, †P < 0.05 vs. baseline. Group C: The group injected with 0.9% normal saline. Group D: The group injected with dexmedetomidine 1 μg/kg. MBP: Mean blood pressure.
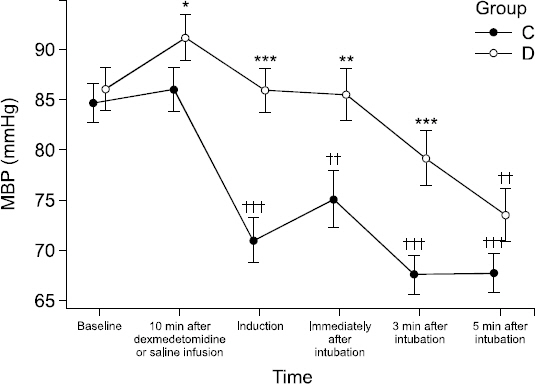
In Group C, the mean arterial pressure (MAP) was significantly decreased at the times after the induction of anesthesia, immediately after endotracheal intubation, and at 3 and 5 minutes after endotracheal intubation compared to baseline (P < 0.001). In Group D, the MAP was significantly decreased only at 5 minutes after endotracheal intubation compared to the baseline value (P < 0.001). The MAP was significantly lower in Group C than in Group D at 10 minutes after dexmedetomidine administration (P = 0.049), after the induction of anesthesia (P < 0.001), immediately after endotracheal intubation (P = 0.008), and at 3 minutes after endotracheal intubation (P < 0.001) (
Fig. 2).
During the induction of anesthesia, ephedrine was administered to two patients in Group C and one patient in Group D at 3 minutes after endotracheal intubation, and atropine was administered to one patient in Group D at 10 minutes after the administration of dexmedetomidine.
DISCUSSION
The results of the present study showed that dexmedetomidine 1 μg/kg improved the intubating conditions and stabilized hemodynamic changes during endotracheal intubation following anesthetic induction performed using propofol 2 mg/kg and remifentanil 1.5 μg/kg.
The administration of remifentanil together with propofol in the absence of a neuromuscular blocker may have different effects on intubating conditions and result in different adverse effects depending on the remifentanil dose. Stevens reported that, in endotracheal intubation performed using propofol 2 mg/kg and different doses of remifentanil including 1 μg/kg, 2 μg/kg, 3 μg/kg, and 4 μg/kg in the absence of a neuromuscular blocker, the intubating conditions were excellent in 35% of patients at 1 μg/kg, in 75% of patients at 2 μg/kg, and in most patients in 3 μg/kg and 4 μg/kg, while the incidence of hypotension and bradycardia was also increased [
1].
Demirkaya et al. [
7] reported that the effective dose (ED50) of remifentanil at which acceptable intubating conditions were observed in 50% of patients was 1.4 μg/kg during induction of anesthesia with propofol 2 mg/kg without a neuromuscular blocker. The dosage of the low-dose remifentanil in the present study was determined with reference to the previous study of Demirkaya et al. [
7].
The improvement of the intubating conditions by dexmedetomidine observed in the present study may be attributable to the increase of the hypnotic depth, the increased effect of remifentanil, and the blunting of the airway reflex. Le Guen et al. [
19] studied the administration of dexmedetomidine in combination with propofol and reported that dexmedetomidine decreased the propofol requirement. Various other researchers have also reported that dexmedetomidine decreased the MAC of inhalational anesthetics [
8,
20,
21].
There are also other reports demonstrating the opioid-sparing effect of dexmedetomidine. It is known that dexmedetomidine significantly enhances the analgesic effect of opioid and thus decreases the opioid requirement [
22,
23]. In addition, there is a report that dexmedetomidine has the effect of preventing muscular stiffness, which is one of the adverse effects of opioids [
24].
Such effects of dexmedetomidine may prevent muscle rigidity caused by bolus injection of high-dose opioid during an endotracheal intubation performed without the administration of a neuromuscular blocker, and thus reduce the risk of hypoxemia.
Hanci et al. [
25] reported that the incidence of airway reflexes, such as coughing, and diaphragmatic movement during endotracheal intubation without a neuromuscular blocker was lower in patients administered propofol and dexmedetomidine than in patients administered propofol and fentanyl. In addition, it has been found in animal and human studies that α2 agonists such as dexmedetomidine attenuate airway constriction [
26,
27].
Although it was reported that dexmedetomidine may cause hypotension at a low loading dose, and hypertension and bradycardia at a high loading dose, no hemodynamic changes were observed when dexmedetomidine was administered slowly over 10 minutes at a low loading dose of 1 μg/kg [
28].
Regarding the hemodynamic changes found in the two groups in the present study, Group C displayed a 16% decrease in MAP after administration of the induction agent, while there were no changes from baseline in Group D. Group C also showed a 20% decrease in MAP at 3 and 5 minutes after endotracheal intubation, while Group D showed 10% and 15% decreases in MAP at 3 and 5 minutes, respectively, after endotracheal intubation. These findings are consistent with previous studies reporting that dexmedetomidine contributes to hemodynamic stability during anesthetic induction, a result also observed in the present study [
29,
30].
In conclusion, dexmedetomidine 1 μg/kg improved the intubating conditions and stabilized hemodynamic changes in endotracheal intubation following anesthetic induction performed using propofol 2 mg/kg and remifentanil 1.5 μg/kg without a neuromuscular blocker.










