MATERIALS AND METHODS
This randomized and double-blind study was conducted with approval of our institutional research ethics board (no. 2015-05-008), having been listed with the Center of Clinical Trials Clinical Registry (no. NCT02969239). Inclusion criteria were American Society of Anesthesiologists class I and II, 18-40 years old parturients, singleton term pregnancy (gestational age > 37 weeks), and anticipated spinal anesthesia for elective cesarean delivery. Exclusion criteria were hypertension, cerebrovascular or cardiovascular disease, any contraindication to spinal anesthesia, allergy to any study medications, fetal anomalies, weight < 50 kg or > 100 kg, and height < 140 cm or > 180 cm.
A total of 56 parturients granted written informed consent to participate and were randomly assigned to one of two groups (phenylephrine vs. norepinephrine) using a computer-generated random number table. Group placements were consigned to sealed, opaque envelopes upon initial randomization. Our research assistant, having no role in clinical patient care or data assessment, opened the sealed envelopes and prepared assigned solutions as instructed. The vasopressors were prepared in 10-ml syringes containing either phenylephrine (100 µg/ml) or norepinephrine (5 µg/ml) and labeled “study drug”. All patients and any physicians involved in clinical patient care were blinded to group allocation.
Arriving at the operating room, patients were placed in left-tilted supine position, and standard monitoring devices (electrocardiography, pulse oximetry, and noninvasive blood pressure gauge) were attached. Four non-invasive CO monitoring (NICOM, Cheetah Medical Inc., USA) sensors were applied to the patient’s chest by a single operator. NICOM provided continuous CO, SV, and HR recordings and 1-min interval assessments of BP and TPR until time of delivery. Baseline SBP was measured by averaging three readings 1 min apart, after which 80% and 120% of mean baseline values were calculated.
Once baseline data were recorded, patients were placed in right lateral position. After skin disinfection and local infiltration (1% lidocaine), spinal anesthesia was induced at L2-3 or L3-4 intervertebral space, using a 27-gauge pencil-point spinal needle (needle-through-needle technique). Having established free flow of cerebrospinal fluid, intrathecal injection of hyperbaric bupivacaine (8 mg) with fentanyl (15 µg) proceeded. Rapid intravenous infusion of lactated Ringer’s solution (10 ml/kg) was instituted immediately after intrathecal injection and later slowed to a maintenance rate. After securing the epidural catheter, the patient was returned to supine position with left uterine displacement, using a wedge under the right hip. Supplemental oxygen (6 L/min) was administered via facial mask. Five min after intrathecal injection, the highest level of sensory block was assessed using an alcohol swab. Patients with upper sensory blocks below T6 level were considered failures and were dropped from the study.
Hypotension was defined as < 80% baseline SBP or < 90 mmHg. An intermittent bolus (1 ml) of study drug was administered manually by the attending anesthesiologist whenever hypotension occurred, using the port nearest to the patient each time. Bolus dosing was repeated at 1-min intervals if hypotension persisted. However, after two consecutive doses, the attending anesthesiologist would revert to standard practice, administering phenylephrine (100 µg) or ephedrine (5 mg) as appropriate to treat persistent hypotension. Bradycardia, defined as HR < 60 beats/min, was treated by intravenous atropine (0.5 mg). Continuously monitored values, such as HR, oxygen saturation, CO, and SV, were recorded at the time of completing each BP determination. Hemodynamic parameters (HR, SBP, CO, SV, and TPR) were recorded at four points in time: baseline (T0), 5 min after intrathecal injection (T1), surgical incision (T2), and baby delivery (T3).
Demographics, intraoperative parameters (intravenous fluid volume, urine output, blood loss, administered vasopressor, anesthetic induction to delivery interval, skin incision to delivery interval), and maternal side effects (bradycardia, nausea, vomiting) were recorded.
Following delivery, carbetocin (100 µg) was administered as a bolus over a 1-min period. Neonatal outcomes were assessed by Apgar scores at 1 and 5 min post-delivery and by umbilical arterial (UA) blood gas analysis, obtaining samples from double-clamped segments of umbilical cord. The study protocol ended 5 min after delivery. Standard delivery of phenylephrine (100 µg) or ephedrine (5 mg) was then at the discretion of the attending anesthesiologist.
Statistical analysis
Group-wise difference in normalized CO served as the primary outcome measure, examining SV, TPR, HR, adverse events (bradycardia, nausea/vomiting), Apgar scores (at 1 and 5 min), and UA blood gas analysis secondarily. In preliminary data, CO (T1) of the phenylephrine group showed a mean of 6.2 ± 1.4 L/min. Our calculations indicated a need of 21 patients in each group to detect 20% difference in CO at 0.05 α-error probability and 90% power. Considering dropouts, the sample size was increased by 20% giving a final sample size of 25 patients per group.
All data were expressed individually as mean ± standard deviation, median (1Q, 3Q), or frequency (%). Continuous data were assessed for normal distribution using the Kolmogorov-Smirnov test, thereafter applying Student’s t-test or Mann-Whitney U test as appropriate for intergroup comparisons. Categorical data were assessed via chi-square or Fisher’s exact test. For within- and between-group comparisons, hemodynamic variables were tested for sphericity (Mauchly’s test) and compared using repeated-measures ANOVA (to check variance and symmetry) and Geisser-Greenhouse correction. In post-hoc analysis, the paired-sample Wilcoxon test was invoked, with Bonferroni correction. All computations relied on standard software (SPSS version 20, IBM Co., USA), setting significance at P < 0.05.
RESULTS
Patient recruitment and flow are summarized in
Fig. 1. From October 2016 through September 2017, 56 patients qualified for enrollment. Ten patients did not receive vasopressors, and two patients were not monitored due to equipment failure, so they were excluded. All patients had undergone successful spinal anesthesia, with sensory block above T6 level. There were 22 patients per group for analysis. Aside from volumes of study drugs used (P = 0.043), the two groups were similar in terms of demographics, perioperative data, and maternal outcomes (
Table 1).
Table 1
Demographic Characteristics, Perioperative Data, and Maternal Outcomes
|
Variable |
Phenylephrine group (n = 22) |
Norepinephrine group (n = 22) |
P value |
|
Age (yr) |
34.7 ± 3.3 |
35.4 ± 3.9 |
0.567 |
|
Body mass index (kg/m2) |
27.4 ± 4.0 |
29.7 ± 9.8 |
0.322 |
|
Height (cm) |
162.1 ± 4.3 |
159.6 ± 4.8 |
0.073 |
|
Weight (kg) |
72.4 ± 10.5 |
68.7 ± 12.9 |
0.418 |
|
Gestational age (wk) |
37.7 (37.4, 38.3) |
37.5 (37.3, 37.9) |
0.188 |
|
Block height at 5 min (T3/T4/T5) |
3/16/3 (13.5/73/13.5) |
9/10/3 (41/45/14) |
0.111 |
|
Spinal anesthesia to delivery interval (min) |
22 (20, 25) |
22 (21, 25) |
0.580 |
|
Skin incision to delivery interval (min) |
6 (5, 8) |
6 (5, 8) |
0.714 |
|
Infused volume of crystalloid before delivery (ml) |
1,019.5 ± 255.6 |
975.0 ± 224.0 |
0.550 |
|
Administered volume of study drug (ml) |
2 (2, 3) |
3 (2, 4) |
0.043 |
|
Bradycardia |
7 (32) |
5 (23) |
0.736 |
|
Nausea/vomiting |
2 (9) |
1 (4.5) |
1.000 |
Fig. 1
CONSORT diagram showing patient recruitment and flow.
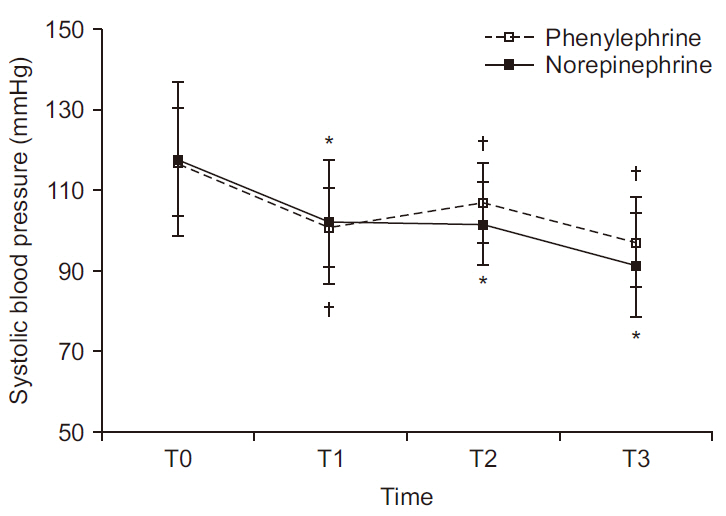
Serial change in SBP is depicted in
Fig. 2. There was no significant difference between groups (P = 0.175); but within-group values differed significantly over time (P < 0.001). In the phenylephrine group, mean SBP (mmHg) (T0, 117; T1, 101; T2, 107; T3, 97) differed significantly at T1, T2, and T3 relative to T0 (P < 0.001, P = 0.008, and P < 0.001, respectively). Likewise, mean SBP (T0, 118; T1, 102; T2, 102; T3, 91) in the norepinephrine group differing significantly at T1, T2, and T3 relative to T0 (P < 0.001, P < 0.001, and P < 0.001, respectively).
Fig. 2
Serial changes in systolic blood pressure at four time periods. There is no difference between the two groups (P = 0.175); whereas there is within-group difference (P < 0.001). Values are presented as mean and standard deviation. T0: baseline, T1: 5 min after intrathecal injection, T2: start surgery, T3: delivery. *P < 0.05 vs. T0 in norepinephrine group. †P < 0.05 vs. T0 in phenylephrine group.
Serial change in HR is depicted in
Fig. 3. There was no significant difference between groups (P = 0.622); but within-group values differed significantly over time (P < 0.001). In the phenylephrine group, median HR (beats/min) (T0, 84; T1, 83; T2, 81; T3, 79) differed significantly at T2 and T3 relative to T0 (P = 0.022 and P = 0.004, respectively). However, median HR recordings in the norepinephrine group (T0, 85; T1, 83; T2, 83; T3, 81) were not significantly different at the four stated time points.
Fig. 3
Serial changes in heart rate at four time periods. There is no difference between the two groups (P = 0.622); whereas there is withingroup difference (P < 0.001). In post-hoc analysis, as heart rate had not shown normal distribution, Mann-Whitney U test for intergroup comparisons and the paired-sample Wilcoxon test for within-group comparisons were invoked, with Bonferroni correction. Values are presented as median and interquartile range. T0: baseline, T1: 5 min after intrathecal injection, T2: start surgery, T3: delivery. *P < 0.05 vs. T0 in norepinephrine group. †P < 0.05 vs. T0 in phenylephrine group.

Trends of normalized CO, SV, and TPR are shown in
Fig. 4. The primary outcome of this study was maternal normalized CO. Normalized CO and SV differed significantly between groups (P < 0.001 and P = 0.002, respectively). In the phenylephrine group, median of normalized CO at T1, T2, and T3 declined relative to CO at T0 (94%, 89%, and 87%, respectively) and differed significantly at T2 and T3 relative to T0 (P < 0.001 and P < 0.001, respectively); whereas in the norepinephrine group, median of normalized CO at T1, T2, and T3 maintained the CO at T0 (104%, 99%, and 100%, respectively). The same was true for median of normalized SV (phenylephrine: T1, 94%; T2, 91%; T3, 95% vs norepinephrine: T1, 103%; T2, 102%; T3, 105%).
Fig. 4
(A) Normalized CO. There is significant difference between groups (P < 0.001; overall). In the phenylephrine group, mean of normalized CO at T1, T2, and T3 declined relative to CO at T0 (94%, 89%, and 87%, respectively) and differed significantly at T2 and T3 relative to T0 (P < 0.001 and P < 0.001, respectively); whereas in the norepinephrine group, mean of normalized CO at T1, T2, and T3 maintained the CO at T0 (104%, 99%, and 100%, respectively). (B) Normalized SV. There is significant difference between groups (P = 0.002, overall), whereas there is no difference within group (P = 0.238, overall). (C) Normalized TPR. There is no difference between groups (P = 0.726, overall). In the phenylephrine group, normalized CO are differed at T1 and T3 relative to T0 (P < 0.001, overall). Values are presented as mean and standard deviation. Normalized value = measured value / baseline value × 100 (%). CO: cardiac output, SV: stroke volume, TPR: total peripheral resistance, T0: baseline, T1: 5 min after intrathecal injection, T2: start surgery, T3: delivery. *P < 0.05 vs. T0 in norepinephrine group. †P < 0.05 vs. T0 in phenylephrine group. ‡P < 0.05 between groups.

With respect to normalized TPR, there was no significant difference between groups (P = 0.726), although within-group normalized TPR values differed significantly (P < 0.001,
Fig. 4C). In the phenylephrine group, mean values of normalized TPR (T1, 84%; T2, 93%; T3, 77%) declined significantly at T1 and T3 relative to baseline (P = 0.014 and P = 0.012, respectively). The norepinephrine group showed a similar trend (T1, 77%; T2, 90%; T3, 76%), with significant declines in mean normalized TPR at T1 and T3 relative to baseline (P = 0.001 and P = 0.019, respectively).
Fetal outcomes are summarized in
Table 2.
Table 2
|
Variable |
Phenylephrine group (n = 22) |
Norepinephrine group (n = 22) |
P value |
|
Sex (M/F) |
13/9 (59/41) |
12/10 (54/46) |
1.000 |
|
Birth weight (kg) |
3.0 (2.8, 3.2) |
3.0 (2.8, 3.1) |
0.993 |
|
Apgar score at 1 min < 7 |
2 (9) |
4 (18) |
0.664 |
|
Apgar score at 5 min < 7 |
0 |
0 |
1.000 |
|
Umbilical arterial blood gases |
|
pH |
7.32 (7.30, 7.33) |
7.31 (7.29, 7.32) |
0.383 |
|
PCO2 (mmHg) |
49.23 (46.97, 51.48) |
51.35 (48.69, 54.00) |
0.212 |
|
PO2 (mmHg) |
19.63 (18.14, 21.13) |
18.21 (16.23, 20.19) |
0.238 |
|
Base excess (mM) |
−1.73 (−2.31, −1.15) |
−1.83 (−2.53, −1.13) |
0.819 |
DISCUSSION
This is the first study to compare the systemic hemodynamic effects of norepinephrine and phenylephrine as intermittent bolus treatment during cesarean delivery. Findings herein underscore the superiority of norepinephrine in maintaining CO and SV when given in this manner under spinal anesthesia.
The present study differs from the recent report by Ngan Kee [
5] analyzing dose-response effects of single-bolus vasopressor use for first episode of hypotension. In this trial, their primary outcome was performance error which was based on recovery of SBP. Subsequent bolus dosing was not pursued, and neither systemic vascular responses nor cardiac parameters were examined. Our analysis actually addressed the systemic hemodynamic effects of phenylephrine and norepinephrine, which were administered as intermittent bolus throughout cesarean delivery, measuring cardiac parameters via NICOM.
During cesarean deliveries, the typical hemodynamic response to spinal anesthesia is diminished systemic vascular resistance, with compensatory increases in HR and CO [
6]. However, our patients consistently showed significant sequential declines in HR and TPR upon administering either phenylephrine or norepinephrine after combined spinal-epidural (CSE). There were no significant group-wise differences in HR and TPR at any of the time points monitored. Contrary to a previous report [
1], which has established elevations of HR and CO with norepinephrine use, we observed significant group differences in CO and SV but no significant differences in HR, SBP, and TPR for the two groups. Although CO and SV were maintained in the norepinephrine group, the phenylephrine group showed reductions of up to 14% in CO and 10% in SV. Based on our results, the enhanced CO of the norepinephrine group may be primarily related to a bolstering of SV.
Phenylephrine effectively treats hypotension in this setting by increasing systemic vascular resistance. It is a pure α-adrenergic agonist, lacking β-adrenergic effects, and may significantly lower (up to 20%) both HR and CO. Because norepinephrine has both α-adrenergic and weak β-adrenergic activity, it may produce vasoconstriction and some inotropic effects, improving cardiac contractility through β1-adrenergic receptor stimulation. Hamzaoui et al. [
7] have shown that early use of norepinephrine during septic shock increases cardiac systolic function, despite a presumptive increase in left ventricular afterload. They have also confirmed that the resultant improvement in cardiac systolic function boosted both SV and CO, without increasing HR.
The disparate results of previous studies (compared with ours) may be explained by differences in modes of anesthesia, vasopressor regimens (continuous vs. intermittent bolus infusion), or total vasopressor dosing. First, in reviewing past study protocols [
1,
3,
8], others have used spinal anesthesia for cesarean deliveries, infusing distinctly higher doses of bupivacaine (11-15 mg) and fentanyl (15-20 µg), whereas we used CSE anesthesia, delivering bupivacaine (8 µg) and fentanyl (15 µg) at lower doses. At relatively higher doses of local anesthetic, spinal anesthesia is known to elicit more profound systemic hemodynamic fluctuations [
6]. Second, we have further noted that in most prior studies [
1,
3,
8], continuous infusions of phenylephrine or norepinephrine were used (39-50 µg/min and 2.4-2.5 µg/min, respectively), resulting in higher total doses than we ordinarily give (phenylephrine, 1,000-1,400 µg; norepinephrine, 47-85 µg vs. phenylephrine, 100-400 µg; norepinephrine, 5-25 µg).
Based on a recent dose-finding trial by Onwochei et al. [
4], the suggested intermittent bolus dose of norepinephrine in this context is 6 µg (ED
90: 5.8 µg, 95% confidence interval 5.01-6.59), which is equivalent to 100 µg of phenylephrine (16:1 potency ratio). However, other reports [
1,
3] cite a potency ratio of 20:1, based on study of the human saphenous vein [
9]. At our institution, 100 µg of phenylephrine is the standard dose for spinal anesthesia-induced hypotension during cesarean delivery; and 5 µg of norepinephrine is our choice for intermittent bolus dosing, given potential hazards of peripheral norepinephrine infusion and various safety issues in parturients. This dose confers a potency ratio of 20:1. In actuality, the true potency ratio of these vasopressors has yet to be confirmed in parturients.
Earlier research [
1,
3,
8] has relied on various technologies for systemic hemodynamic monitoring, perhaps accounting for some differences in our findings. We used a NICOM system based on bioreactance technology to measure systemic hemodynamic variables throughout the study period. These variables are known to correlate well with those derived by pulmonary artery catheter [
10]. NICOM has been validated in parturients and has demonstrated acceptable accuracy, precision, and responsiveness in patient monitoring over a wide range of circulatory disturbances [
11]. It has recently been introduced in obstetric settings.
Although peripheral infusion of norepinephrine does raise concerns [
12,
13], a dose of 5 µg/ml is roughly equivalent in vasoconstrictor potency to 100 µg/ml of phenylephrine[
9]. Hence, the ischemic risk of norepinephrine would be no greater than that of phenylephrine when used at accepted standard concentrations. Both drugs should always be delivered via larger-bore cannula, confirming proper intravenous line function beforehand and constantly flushing [
14]. We administered small, intermittent bolus doses, flushing the line with 5 ml of saline. And in our institution, all patients are sedated after meeting her baby and complaints about extravasation are immediately possible. Tissue ischemic complications, resulted from peripheral administration of vasopressors, were not observed throughout present study.
Our study has certain limitations. Although fetal outcomes did not differ significantly, there are lingering safety issues for the fetus and neonate when administering norepinephrine. In general, the placental capacity to degrade catecholamines makes it unlikely that norepinephrine will readily cross this barrier [
15]. We did not measure umbilical or fetal catecholamine levels, and all study subjects were healthy parturients undergoing elective cesarean delivery, so we cannot comment on the safety of norepinephrine use in compromised parturients, states of uteroplacental insufficiency, or growth-restricted fetuses. More data is needed on umbilical norepinephrine concentrations and comparative norepinephrine/phenylephrine use in this setting to establish safety profiles and therapeutic indications.
Another issue is whether the β-adrenergic effects of norepinephrine actually bear clinical relevance [
13]. In a compromised mother or fetus, this issue is likely to assume greater importance than in healthy subjects, although in these situations the norepinephrine use has not been established. Further studies are needed to establish the clinical benefits of norepinephrine administration in circumstances of fetal compromise or uteroplacental insufficiency.
In conclusion, our findings serve to validate the use of norepinephrine in intermittent bolus doses to treat spinal anesthesia-induced hypotension during elective cesarean delivery. Further studies are warranted to clarify whether the cardiovascular advantages of norepinephrine over phenylephrine translate into improved clinical outcomes in a compromised fetus.









