INTRODUCTION
Diabetes mellitus (DM) is the most representative metabolic disorder with increasing prevalence. End-stage liver disease (ESLD) commonly causes metabolic disorders, especially disorders of glucose metabolism such as DM [
1]. Furthermore, there has been a rapid increase in the incidence of ESLD resulting from non-alcoholic fatty liver disease, which is related to metabolic disorder. Therefore, the number of liver transplantation due to non-alcoholic fatty liver disease has increased in recent years [
2,
3]. As a result, there is increased interest in the effect of the interaction between disorders of glucose metabolism (such as DM) and ESLD [
4,
5].
The chronic cardiac dysfunction typically seen in ESLD is called cirrhotic cardiomyopathy. Increased baseline stroke volume (SV), decreased systemic vascular resistance, and increased heart rate are characteristics of this condition. The sympathetic nervous system becomes more active as baseline hepatic dysfunction worsens, thus increasing the baseline systolic function. However, collagen deposition within the myocardium causes left ventricular hypertrophy and increased myocardial stiffness, thereby decreasing the diastolic function [
6]. Recently, the association between changes in cardiac function, especially diastolic dysfunction, and the prognosis of ESLD patients undergoing liver transplant has been actively investigated [
6-
8].
DM shows a high correlation with cardiac failure. DM-related cardiomyopathy (DM-CMP) is a known chronic cardiac disorder that characteristically occurs in patients with DM [
9]. DM-CMP shows characteristics such as preceding diastolic dysfunction, systolic dysfunction, and left ventricular hypertrophy. DM-CMP is known to be caused by DM-related metabolic disorders, myocardial fibrosis, small vessel disease, cardiac autonomic neuropathy, and insulin resistance [
10].
However, there is limited research on the difference in cardiac systolic or diastolic dysfunction between ESLD patients with DM (DM-ESLD) and ESLD patients without DM (Non DM-ESLD). Therefore, in this study, we aimed to evaluate the preoperative cardiac echocardiography data of liver transplant recipients to compare the systolic function and diastolic function in those with and without DM.
MATERIALS AND METHODS
This study retrospectively analyzed the echocardiography results of 1,572 adult ESLD patients who underwent liver transplant between January 2012 and June 2016. This study was conducted with approval from the Asan Medical Center Bioethics Committee (no. 2019-0107) and according to the ethical principles for medical research summarized in the 1975 Helsinki Declaration. Patients in whom DM was diagnosed by an endocrinologist before liver transplant and those with fasting glucose Ōēź 126 mg/dl, random glucose Ōēź 200 mg/dl, glucose level Ōēź 200 mg/dl at 2 h after the oral administration of 75 g glucose, or hemoglobin A
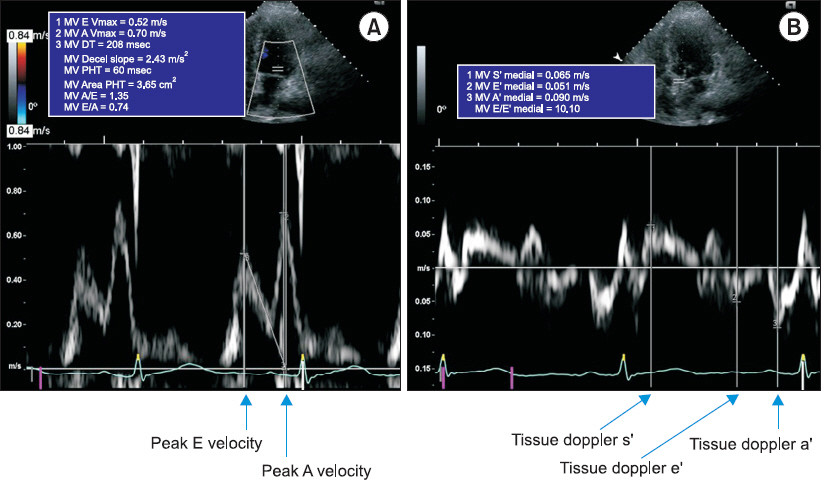
1c Ōēź 6.5% were classified as having DM. Those without any diagnostic test results were classified into the control group. The severity of ESLD was evaluated using the Model for End-stage Liver Disease (MELD) score based on serum bilirubin, creatinine, and international normalized ratio. Patients aged < 18 years, with a history of liver transplant, with concomitant chronic kidney disease, with a history of cardiac surgery or moderate to severe valvular disease, and without a preoperative echocardiogram were excluded. Demographic data, patient status before liver transplant, blood test results, and hemodynamic data were collected from electronic medical records. Transthoracic echocardiography was performed by a skilled technician using a Hewlett-Packard Sonos 2500 or 5500 imaging system (Hewlett-Packard Inc., USA) with a 2.5-MHz transducer, and the measurements were confirmed by a cardiologist. The 2-dimensional variables measured included end-systolic interventricular diameter, end-diastolic interventricular diameter, end-systolic left ventricular posterior wall thickness, end-diastolic left ventricular posterior wall thickness, left ventricular mass index, and left atrial diameter. The Teichholz method or biplane modified SimpsonŌĆÖs rule was used as appropriate to measure the left ventricular end-diastolic volume (EDV) and left ventricular end-systolic volume (ESV), which were used to calculate the SV (= EDV ŌłÆ ESV) and left ventricular ejection fraction (LVEF). The index of each measured value was calculated by dividing each value by the body surface area. In the apical 4-chamber view, pulsed-wave Doppler was used to measure early mitral inflow velocity (E), late mitral inflow velocity (A), deceleration time of the E wave, and E/A ratio. The tissue Doppler image was used to measure the velocities of systolic wave (sŌĆÖ), early diastolic wave (eŌĆÖ), and late diastolic wave (aŌĆÖ) on the luminal side of the septal mitral annulus. The E/eŌĆÖ ratio, which reflects the left ventricular end-diastolic pressure, was calculated (
Fig. 1). For the evaluation of more complicated left ventricular end-systolic function, end-systolic elastance was calculated as ESV / end-systolic pressure. End systolic pressure, a reflection of aortic pressure, was calculated as noninvasive systolic pressure ├Ś 0.9. To evaluate vascular resistance, arterial elastance was calculated as end-systolic pressure / SV [
11].
Fig.┬Ā1
Representative of echocardiographic measurement of (A) transmitral peak E velocity, peak A velocity and (B) tissue doppler image of sŌĆÖ, eŌĆÖ and aŌĆÖ velocity. DT: deceleration time, PHT: pressure half-time.
All values were expressed as mean ┬▒ standard deviation, median (1st quartile, 3rd quartile), or number of patients (percent). Continuous variables were analyzed using the Shapiro-Wilk normality test and subsequent StudentŌĆÖs
t-test or Mann-Whitney test, as appropriate. Categorical variables were analyzed using the chi-square test or FisherŌĆÖs exact test. To minimize the difference in baseline characteristics of the 2 groups, a 1:2 matched propensity score analysis [
12] was performed. The propensity score, which is the probability of each subject to be assigned to the treatment group according to a given covariance, was calculated using a propensity score model via logistic regression analysis with the patientŌĆÖs age, sex, body mass index, MELD score, hypertension, history of cardiovascular disease, history of beta-blocker use, and btype natriuretic protein, among the characteristics specified in
Table 1. Caliper matching was performed in a 1:2 ratio by using this propensity score in the nonrandom package (
http://www.rdocumentation.org/packages/nonrandom) of R, and the caliper was set as standard deviation*0.2. The model test for propensity scores was performed using Cstatistics and the Hosmer-Lemeshow test. R (version 3.3.1; R Foundation for Statistical Computing, Austria) was used for statistical analysis, and paired
t-test was used to analyze the difference between the 2 matched groups. P < 0.05 was deemed statistically significant.
Table┬Ā1
Demographic of Enrolled Liver Transplant Recipients, Compared according to Prevalence of Diabetes
|
Variable |
Without diabetes (n = 1,007) |
Diabetes (n = 312) |
P value |
SMD |
|
Age (yr) |
53 (48, 58) |
55 (51, 61) |
< 0.001 |
0.383 |
|
Sex (male) |
742 (74) |
246 (79) |
0.078 |
0.122 |
|
Body mass index (kg/m2) |
23.6 (21.6, 25.6) |
23.4 (21.1, 25.6) |
0.340 |
0.062 |
|
Model for end-stage liver disease score |
13 (9, 20) |
12 (9, 18) |
0.213 |
0.180 |
|
Cardiovascular disease |
40 (4) |
29 (9) |
< 0.001 |
0.215 |
|
Hypertension |
129 (13) |
83 (27) |
< 0.001 |
0.352 |
|
Beta blocker use |
200 (20) |
89 (29) |
0.002 |
0.203 |
|
Etiology of liver cirrhosis |
|
|
|
|
|
Hepatitis B virus |
615 (61) |
178 (57) |
0.230 |
0.082 |
|
Hepatitis C virus |
75 (7) |
29 (9) |
0.348 |
0.067 |
|
Alcoholic cirrhosis |
190 (19) |
67 (21) |
0.350 |
0.065 |
|
Biliary disease |
30 (3) |
6 (2) |
0.423 |
0.068 |
|
Other disease |
112 (11) |
42 (13) |
0.306 |
0.071 |
|
Combined hepatocellular carcinoma |
491 (49) |
163 (52) |
0.312 |
0.070 |
|
Laboratory variables |
|
|
|
|
|
Prothrombin time, INR |
1.36 (1.17, 1.76) |
1.36 (1.18, 1.58) |
0.258 |
0.193 |
|
Total bilirubin (mg/dl) |
1.9 (1.0, 5.9) |
1.8 (1.0, 3.8) |
0.116 |
0.258 |
|
Creatinine (mg/dl) |
0.75 (0.63, 0.92) |
0.76 (0.64, 1.00) |
0.286 |
0.006 |
|
B-type natriuretic peptide (pg/ml) |
45 (20, 99) |
49 (25, 104) |
0.352 |
0.019 |
RESULTS
Of 1,572 patients planned for liver transplant, 1,319 met the inclusion criteria. Of these patients, 312 (23.7%) had diabetes.
Table 1 summarizes the demographic data, cause of ESLD, blood test results, hemodynamic status, and echocardiogram results of the DM-ESLD and Non DM-ESLD groups. DM-ESLD patients tended to be older and showed a greater incidence of accompanying cardiovascular disease such as hypertension, coronary artery disease, and cerebrovascular disease than Non DM-ESLD patients. DM-ESLD patients also tended to have a higher frequency of beta-blocker use. On echocardiograms, DM-ESLD patients showed decreased LVEF (64.6 ┬▒ 4.2% vs. 64.0 ┬▒ 4.2%, P = 0.042) and systolic flow velocity (sŌĆÖ) on tissue Doppler (8.4 [7.5-9.5] cm/s vs. 8.2 [7.2-9.1] cm/s, P = 0.003) compared with Non DM-ESLD patients, suggesting decreased left ventricular systolic function. Further, the E/A ratio, a marker for diastolic dysfunction, was lower (1.16 [0.90-1.40] vs. 1.02 [0.84-1.27], P < 0.001); the eŌĆÖ velocity was lower (7.7 [6.5-8.9] cm/s vs. 7.0 [5.9-8.1], P < 0.001); and E/eŌĆÖ, a marker of left ventricular end-diastolic pressure, was higher (9.0 [8.0-12.0] vs. 10.0 [9.0-13.0], P < 0.001) in DM-ESLD patients (
Table 2).
Table┬Ā2
Echocardiography of Enrolled Liver Transplant Recipients, Compared according to Prevalence of Diabetes
|
Variable |
Without diabetes (n = 1,007) |
Diabetes (n = 312) |
P value |
|
Hemodynamic values |
|
ŌĆā Systolic blood pressure (mmHg) |
106 (97, 118) |
106 (98, 116) |
0.943 |
|
ŌĆā Diastolic blood pressure (mmHg) |
67 (61, 75) |
68 (61, 74) |
0.987 |
|
ŌĆā End systolic pressure (mmHg) |
95 (87, 106) |
96 (88, 104) |
0.943 |
|
ŌĆā Arterial elastance |
1.38 (1.12, 1.70) |
1.37 (1.12, 1.71) |
0.948 |
|
ŌĆā End-systolic elastance |
2.52 (2.03, 3.16) |
2.45 (2.03, 3.09) |
0.467 |
|
Left ventricular structures and Systolic Functional values |
|
ŌĆā LV dimension in systole (mm) |
30 (27, 33) |
30 (27, 33) |
0.830 |
|
ŌĆā LV dimension in diastole (mm) |
50 (47, 54) |
50 (47, 53) |
0.385 |
|
ŌĆā LV posterior wall thickness in systole (mm) |
14 (13, 15) |
14 (13, 15) |
0.684 |
|
ŌĆā LV posterior wall thickness in diastole (mm) |
9 (8, 10) |
9 (8, 10) |
0.417 |
|
ŌĆā Interventricular septal thickness in systole (mm) |
13 (12, 14) |
14 (12, 15) |
0.137 |
|
ŌĆā Interventricular septal thickness in diastole (mm) |
9 (8, 10) |
9 (8, 10) |
0.472 |
|
ŌĆā Left atrium (mm) |
39 (36, 43) |
39 (36, 43) |
0.190 |
|
ŌĆā Aorta (mm) |
33 (30, 35) |
33 (31, 36) |
0.001 |
|
ŌĆā End-systolic volume (ml) |
39 (31, 46) |
40 (31, 48) |
0.393 |
|
ŌĆā End-diastolic volume (ml) |
109 (90, 132) |
109 (90, 132) |
0.843 |
|
ŌĆā Stroke volume (ml) |
69 (58, 85) |
71 (57, 84) |
0.908 |
|
ŌĆā Stroke volume index |
41 (34, 48) |
41 (34, 48) |
0.830 |
|
ŌĆā LVMI (g/m2) |
88 (76, 102) |
90 (76, 102) |
0.842 |
|
ŌĆā LV ejection fraction (%) |
64.6 ┬▒ 4.2 |
64.0 ┬▒ 4.2 |
0.042 |
|
ŌĆā sŌĆÖ medial (cm/s) |
8.4 (7.5, 9.5) |
8.2 (7.2, 9.1) |
0.003 |
|
Diastolic function and RV function |
|
ŌĆā E/A ratio |
1.16 (0.90, 1.40) |
1.02 (0.84, 1.27) |
|
|
ŌĆā E/A ratio Ōēż 1.0 |
354 (35.2) |
154 (49.4) |
< 0.001 |
|
ŌĆā E/A ratio > 1.0 |
653 (64.8) |
158 (50.6) |
< 0.001 |
|
ŌĆā Peak E velocity (cm/s) |
74 (61, 88) |
72 (62, 85) |
0.268 |
|
ŌĆā Peak A velocity (cm/s) |
64 (54, 76) |
69 (58, 82) |
< 0.001 |
|
ŌĆā Deceleration time (ms) |
210 (183, 238) |
214 (185, 240) |
0.358 |
|
ŌĆā eŌĆÖ medial (cm/s) |
7.7 (6.5, 8.9) |
7.0 (5.9, 8.1) |
< 0.001 |
|
ŌĆā aŌĆÖ medial (cm/s) |
9.4 (8.1, 10.7) |
9.4 (8.1, 10.5) |
0.810 |
|
ŌĆā E/eŌĆÖ |
9.0 (8.0, 12.0) |
10.0 (9.0, 13.0) |
< 0.001 |
|
ŌĆā Peak TR velocity (m/s) |
2.4 (2.2, 2.5) |
2.3 (2.2, 2.5) |
0.411 |
|
ŌĆā RV PGsys (mmHg) |
23 (19, 25) |
21 (19, 25) |
0.411 |
Table 3 shows the results of 1:2 propensity matching, which involved 306 DM-ESLD patients and 607 Non DM-ESLD patients (1:1.98). The C-statistics value of the propensity score model was 0.662, and the calibration by Hosmer-Lemeshow statistics showed Žć
2 = 12.113, df = 8, and P = 0.1462. Baseline variables that showed a difference between the 2 groups before matching showed P > 0.05 after matching (
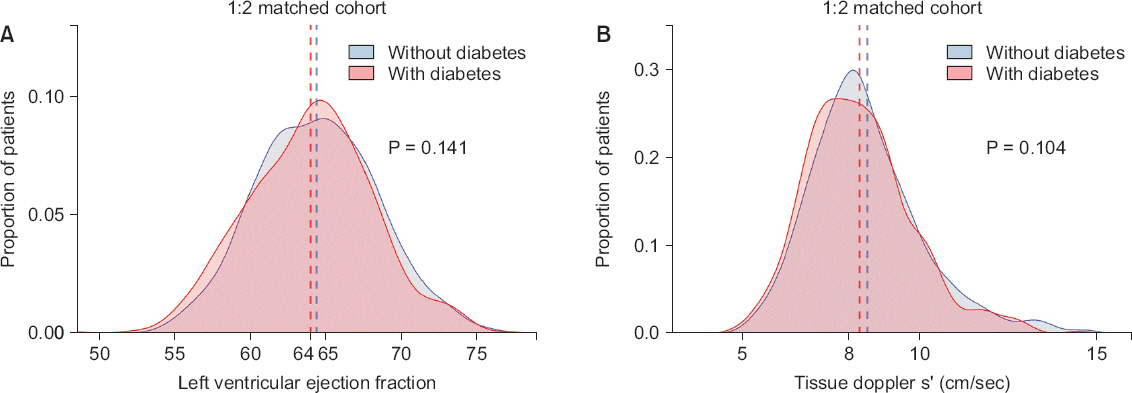
Table 3). On echocardiograms, systolic function indices that were significant before matching (LVEF, sŌĆÖ velocity; both P > 0.05) did not show a significant difference after matching (
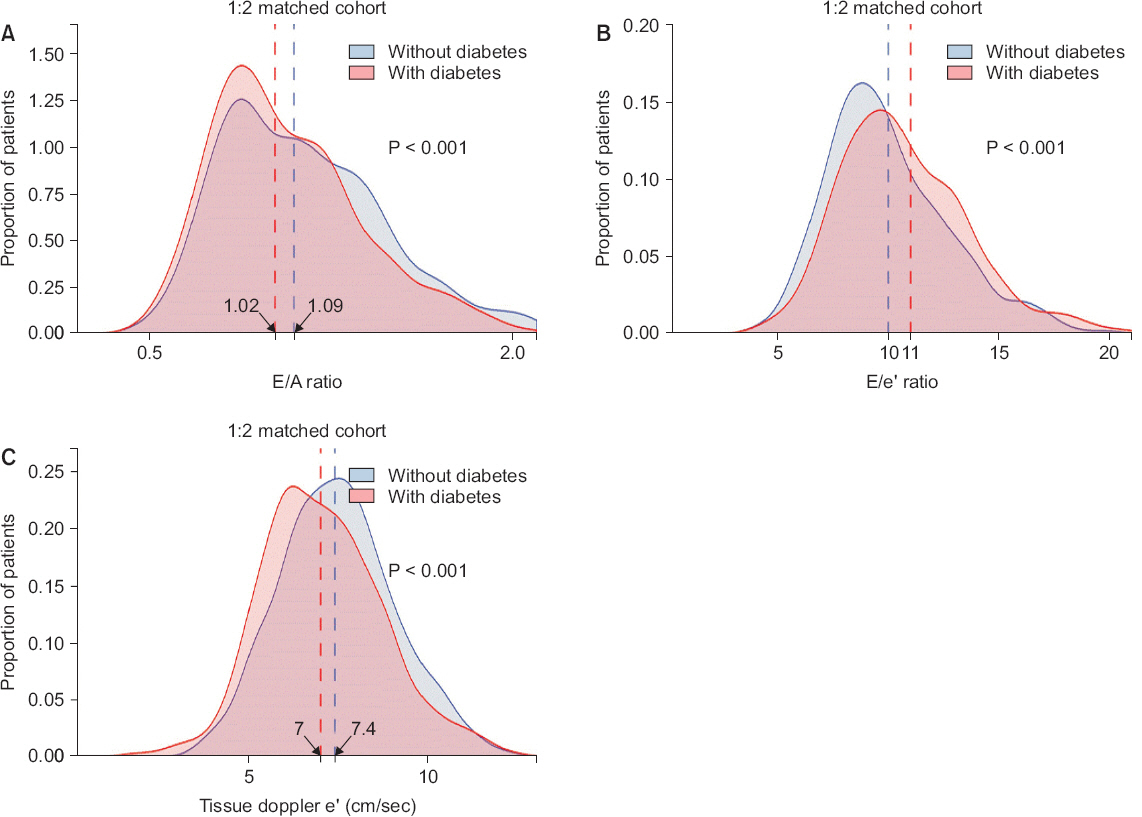
Fig. 2). However, diastolic function indices including E/A ratio (1.09 [0.87-1.37] vs. 1.02 [0.85-1.28], P = 0.017) and eŌĆÖ velocity (7.4 [6.4-8.5] cm/s vs. 7.0 [5.9-8.1] cm/s, P < 0.001) were still low in DM-ESLD patients, whereas the E/eŌĆÖ ratio, a marker of left ventricular end-diastolic pressure, was still high (10.1 ┬▒ 3.0 vs.10.9 ┬▒ 3.2, P < 0.001) (
Table 4,
Fig. 3).
Table┬Ā3
Demographic Liver Transplant Recipients after 1:2 Propensity Score Matching, Compared according to Prevalence of Diabetes
|
Variable |
Without diabetes (n = 607) |
Diabetes (n = 306) |
P value |
SMD |
|
Age (yr) |
55 ┬▒ 6.9 |
55 ┬▒ 7.5 |
0.880 |
0.010 |
|
Sex (male) |
491 (81) |
241 (79) |
0.500 |
0.053 |
|
Body mass index (kg/m2) |
23.5 (21.4, 25.5) |
23.4 (21.0, 25.6) |
0.675 |
0.020 |
|
Model for end-stage liver disease score |
12 (8, 18) |
12 (9, 18) |
0.348 |
0.011 |
|
Cardiovascular disease |
37 (6) |
26 (8) |
0.225 |
0.092 |
|
Hypertension |
125 (21) |
77 (25) |
0.137 |
0.109 |
|
Beta blocker use |
159 (26) |
86 (28) |
0.592 |
0.043 |
|
Etiology of liver cirrhosis |
|
ŌĆā Hepatitis B virus |
377 (62) |
177 (58) |
0.240 |
0.087 |
|
ŌĆā Hepatitis C virus |
56 (9) |
29 (9) |
0.998 |
0.009 |
|
ŌĆā Alcoholic cirrhosis |
116 (19) |
65 (21) |
0.500 |
0.053 |
|
ŌĆā Biliary disease |
11 (2) |
6 (2) |
1.000 |
0.011 |
|
ŌĆā Other disease |
58 (10) |
39 (13) |
0.173 |
0.101 |
|
ŌĆā Combined hepatocellular carcinoma |
323 (53) |
162 (53) |
0.994 |
0.005 |
|
Laboratory variables |
|
ŌĆā Prothrombin time, INR |
1.32 (1.14, 1.60) |
1.36 (1.18, 1.58) |
0.315 |
0.025 |
|
ŌĆā Total bilirubin (mg/dl) |
1.6 (0.9, 4.2) |
1.8 (1.0, 3.9) |
0.553 |
0.112 |
|
ŌĆā Creatinine (mg/dl) |
0.77 (0.66, 0.92) |
0.76 (0.63, 1.00) |
0.854 |
0.014 |
|
ŌĆā B-type natriuretic peptide (pg/ml) |
47 (22, 103) |
49 (23, 104) |
0.956 |
0.053 |
Fig.┬Ā2
Comparison of systolic function in matched set. Density histograms depict frequency of (A) left ventricular ejection fraction and (B) tissue Doppler sŌĆÖ velocity. Vertical dashed lines show mean value for left ventricular ejection fraction and median for tissue doppler sŌĆÖ velocity (both P > 0.1).
Table┬Ā4
Echocardiography of Liver Transplant Recipients after 1:2 Propensity Score Matching, Compared according to Prevalence of Diabetes
|
Variable |
Without diabetes (n = 607) |
Diabetes (n = 306) |
P value |
|
Hemodynamic values |
|
ŌĆā Systolic blood pressure (mmHg) |
106 (97, 117) |
107 (98, 117) |
0.390 |
|
ŌĆā Diastolic blood pressure (mmHg) |
67 (60, 75) |
68 (61, 74) |
0.879 |
|
ŌĆā End systolic pressure (mmHg) |
95 (87, 105) |
96 (88, 105) |
0.390 |
|
ŌĆā Arterial elastance |
1.39 (1.14, 1.70) |
1.37 (1.12, 1.71) |
0.799 |
|
ŌĆā End-systolic elastance |
2.53 (2.04, 3.14) |
2.45 (2.02, 3.09) |
0.477 |
|
Left ventricular structures and Systolic Functional values |
|
ŌĆā LV dimension in systole (mm) |
30 ┬▒ 4 |
30 ┬▒ 5 |
0.303 |
|
ŌĆā LV dimension in diastole (mm) |
50 (47, 53) |
50 (47, 53) |
0.748 |
|
ŌĆā LV posterior wall thickness in systole (mm) |
14 (13, 15) |
14 (13, 15) |
0.911 |
|
ŌĆā LV posterior wall thickness in diastole (mm) |
9 (8, 10) |
9 (8, 10) |
0.826 |
|
ŌĆā Interventricular septal thickness in systole (mm) |
13 (12, 14) |
14 (12, 15) |
0.380 |
|
ŌĆā Interventricular septal thickness in diastole (mm) |
9 (8, 10) |
9 (8, 10) |
0.905 |
|
ŌĆā Left atrium (mm) |
39 (36, 43) |
39 (36, 43) |
0.742 |
|
ŌĆā Aorta (mm) |
33 (30, 35) |
33 (31, 36) |
0.099 |
|
ŌĆā End-systolic volume (ml) |
39 (31, 46) |
40 (31, 48) |
0.251 |
|
ŌĆā End-diastolic volume (ml) |
108 (91, 128) |
109 (90, 132) |
0.387 |
|
ŌĆā Stroke volume (ml) |
68 (58, 84) |
71 (57, 84) |
0.496 |
|
ŌĆā Stroke volume index |
40 (34, 47) |
41 (34, 48) |
0.581 |
|
ŌĆā LVMI (g/m2) |
89 (76, 103) |
90 (76, 102) |
0.995 |
|
ŌĆā LV ejection fraction (%) |
64.4 ┬▒ 4.1 |
64.0 ┬▒ 4.2 |
0.141 |
|
ŌĆā sŌĆÖ medial (cm/s) |
8.3 (7.5, 9.4) |
8.2 (7.2, 9.1) |
0.104 |
|
Diastolic function and RV function |
|
ŌĆā E/A ratio |
1.09 (0.87, 1.37) |
1.02 (0.85, 1.28) |
|
|
ŌĆā E/A ratio Ōēż 1 |
244 (40.2) |
150 (49.0) |
0.017 |
|
ŌĆā E/A ratio > 1 |
363 (59.8) |
156 (51.0) |
0.014 |
|
ŌĆā Peak E velocity (cm/s) |
71 (60, 86) |
72 (62, 85) |
0.475 |
|
ŌĆā Peak A velocity (cm/s) |
64 (54, 76) |
68 (58, 81) |
< 0.001 |
|
ŌĆā Deceleration time (ms) |
214 (186, 244) |
214 (185, 239) |
0.679 |
|
ŌĆā eŌĆÖ medial (cm/s) |
7.4 (6.4, 8.5) |
7.0 (5.9, 8.1) |
< 0.001 |
|
ŌĆā aŌĆÖ medial (cm/s) |
9.4 (8.2, 10.6) |
9.4 (8.1, 10.5) |
0.427 |
|
ŌĆā E/eŌĆÖ |
10.1 ┬▒ 3.0 |
10.9 ┬▒ 3.2 |
< 0.001 |
|
ŌĆā Peak TR velocity (m/s) |
2.4 (2.2, 2.5) |
2.3 (2.2, 2.5) |
0.516 |
|
ŌĆā RV PGsys (mmHg) |
23 (19, 25) |
21 (19, 25) |
0.516 |
Fig.┬Ā3
Comparison of diastolic function in matched set. Density histograms depict frequency of (A) transmitral E/A ratio, (B) E/eŌĆÖ ratio and (C) tissue doppler eŌĆÖ velocity. Vertical dashed lines show median value for E/A ratio and eŌĆÖ velocity while mean values for E/eŌĆÖ ratio (all P < 0.001).
DISCUSSION
The main finding of this study is that DM-ESLD patients showed worse left ventricular diastolic function compared to Non DM-ESLD patients, whereas no difference in left ventricular systolic function was found. While it is normal for ESLD patients to show diastolic relaxation dysfunction, DMESLD patients showing more severe diastolic dysfunction implies that DM exacerbates cardiac diastolic dysfunction in ESLD patients. On the other hand, left ventricular systolic function was slightly worse in Non DM-ESLD patients before matching, but there was no significant difference between the 2 groups after matching for age, sex, beta-blocker use, and other factors.
Recently, the transplant rejection and mortality rates after liver transplant have decreased drastically owing to improvements in surgical skills as well as successful intraoperative anesthetic management. Early complications and death after liver transplant depend largely on the severity of preoperative cirrhotic cardiomyopathy as well as the presence of cardiovascular disease, such as coronary artery disease [
13]. Therefore, preoperative cardiac assessment before liver transplant is crucial and currently, all patients now undergo preoperative echocardiography. In particular, many of the recent studies focused on the cardiac diastolic dysfunction [
6,
14]. According to Mittal et al. [
8], patients with accompanying left ventricular diastolic dysfunction before liver transplant show higher rates of transplant rejection and death [
15]. Therefore, considering these results as well as the results of the current study, the decrease in cardiac function in DM-ESLD patients may exert adverse effects on postoperative cardiac failure and the viability of the transplanted liver. Hence, this topic requires further research.
After DM-CMP was introduced by Lundbeck in 1954 [
16], this condition has been the subject of many studies. Twenty years after the term was coined, Rubler et al. [
17] showed evidence that DM-CMP is directly caused by DM rather than by complications of DM such as coronary artery disease. In other words, the mechanism behind DM-CMP is believed to be microvascular disease and myocardial metabolic dysfunction due to DM. DM-CMP was classified as a dilated phenotype with eccentric left ventricular remodeling and systolic dysfunction, similar to the pathophysiology of dilated cardiomyopathy [
9]. However, with the increased incidence of type 2 DM, elderly, obese, female patients, who comprise the majority of patients with DM, have shown characteristics such as a small left ventricle, thick ventricular walls, a large left atrium, and normal LVEF. In other words, unlike the previously known phenotype, DM-CMP now mainly shows features of a restrictive phenotype with concentric left ventricular remodeling and diastolic dysfunction [
18], similar to those of heart failure with preserved ejection fraction (HFpEF), which account for the majority of heart failure cases [
19].
In summary, the recently emerging restrictive phenotype of DM-CMP tends to manifest similarly to HFpEF [
9]. We predicted that there is a possibility of alleviation of diastolic dysfunction in patients with DM considering that ESLD patients experience cirrhotic cardiomyopathy with increased left ventricular size. However, we found that DM-ESLD patients show exacerbation of diastolic dysfunction. This is probably because DM worsens the cardiac diastolic dysfunction in ESLD patients. In addition, before matching, DM-ESLD patients showed a higher rate of concomitant cardiovascular disease, such as coronary artery disease, than Non DM-ESLD patients. However, in this study, we only analyzed the presence of cardiovascular disease after negating a significant difference with propensity score matching, in order to consider the effect of DM alone.
This study has a few limitations. First, owing to the retrospective nature of this study, the causality between DM and ESLD is unclear. It is not known whether DM developed before ESLD or secondarily according to the progress of ESLD; thus, more detailed research is needed. Second, this is a single-center study and multicenter prospective studies are needed in the future. Third, although E/A ratio < 1 on Doppler echocardiography means mild diastolic dysfunction, E/ A > 1 can either mean normal diastolic function or moderate pseudo-normalization. Although E/A ratio < 1 is specified in the 2005 definition of cirrhotic cardiomyopathy, the E/A ratio alone is not enough as a criterion for diastolic dysfunction and other markers of diastolic dysfunction are needed. Fourth, cardiac diastology is not yet well established and new guidelines on grading have been published by American and European cardiology societies [
20,
21]. In the future, studies that compare surgical outcomes using these guidelines would be necessary. Fifth, this study showed that DM-ESLD patients have a higher incidence of diastolic dysfunction, but the mechanism and pathophysiology behind this observation have not been studied. More research is needed on this topic.
In conclusion, in the comparison of the systolic and diastolic function of DM-ESLD and Non DM-ESLD patients based on preoperative echocardiograms, DM-ESLD patients showed similar systolic function but worse diastolic function. Therefore, preservation of postoperative cardiac function and the transplanted liver is essential in DM-ESLD patients, considering that ESLD patients with accompanying left ventricular diastolic dysfunction showed higher mortality and transplant rejection rates. Further prospective studies are needed on this topic.












